Elastin fragments drive disease progression in a murine model of emphysema
- PMID: 16470245
- PMCID: PMC1361346
- DOI: 10.1172/JCI25617
Elastin fragments drive disease progression in a murine model of emphysema
Abstract
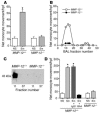
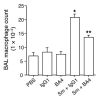
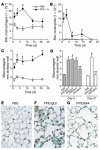
Mice lacking macrophage elastase (matrix metalloproteinase-12, or MMP-12) were previously shown to be protected from the development of cigarette smoke-induced emphysema and from the accumulation of lung macrophages normally induced by chronic exposure to cigarette smoke. To determine the basis for macrophage accumulation in experimental emphysema, we now show that bronchoalveolar lavage fluid from WT smoke-exposed animals contained chemotactic activity for monocytes in vitro that was absent in lavage fluid from macrophage elastase-deficient mice. Fractionation of the bronchoalveolar lavage fluid demonstrated the presence of elastin fragments only in the fractions containing chemotactic activity. An mAb against elastin fragments eliminated both the in vitro chemotactic activity and cigarette smoke-induced monocyte recruitment to the lung in vivo. Porcine pancreatic elastase was used to recruit monocytes to the lung and to generate emphysema. Elastin fragment antagonism in this model abrogated both macrophage accumulation and airspace enlargement.
Figures
Similar articles
-
Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice.Science. 1997 Sep 26;277(5334):2002-4. doi: 10.1126/science.277.5334.2002. Science. 1997. PMID: 9302297
-
Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice.Am J Respir Cell Mol Biol. 2002 Sep;27(3):368-74. doi: 10.1165/rcmb.4791. Am J Respir Cell Mol Biol. 2002. PMID: 12204900
-
The role of matrix metalloproteinase-9 in cigarette smoke-induced emphysema.Am J Respir Crit Care Med. 2011 Apr 1;183(7):876-84. doi: 10.1164/rccm.201005-0718OC. Epub 2010 Nov 5. Am J Respir Crit Care Med. 2011. PMID: 21057003 Free PMC article.
-
Increased surfactant protein-D and foamy macrophages in smoking-induced mouse emphysema.Respirology. 2007 Mar;12(2):191-201. doi: 10.1111/j.1440-1843.2006.01009.x. Respirology. 2007. PMID: 17298450 Review.
-
Matrix metalloproteinases in emphysema.Matrix Biol. 2018 Nov;73:34-51. doi: 10.1016/j.matbio.2018.01.018. Epub 2018 Mar 23. Matrix Biol. 2018. PMID: 29406250 Free PMC article. Review.
Cited by
-
The "Elastic Perspective" of SARS-CoV-2 Infection and the Role of Intrinsic and Extrinsic Factors.Int J Mol Sci. 2022 Jan 29;23(3):1559. doi: 10.3390/ijms23031559. Int J Mol Sci. 2022. PMID: 35163482 Free PMC article. Review.
-
Pathological observation of the effects of exposure to radioactive microparticles on experimental animals.J Radiat Res. 2022 Aug 13;63(Supplement_1):i26-i37. doi: 10.1093/jrr/rrac045. J Radiat Res. 2022. PMID: 35968993 Free PMC article. Review.
-
Epithelial cell apoptosis causes acute lung injury masquerading as emphysema.Am J Respir Cell Mol Biol. 2009 Oct;41(4):407-14. doi: 10.1165/rcmb.2008-0137OC. Epub 2009 Feb 2. Am J Respir Cell Mol Biol. 2009. PMID: 19188661 Free PMC article.
-
Modification and functional inactivation of the tropoelastin carboxy-terminal domain in cross-linked elastin.Matrix Biol. 2008 Sep;27(7):631-9. doi: 10.1016/j.matbio.2008.06.001. Epub 2008 Jun 17. Matrix Biol. 2008. PMID: 18602002 Free PMC article.
-
Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease.Int J Chron Obstruct Pulmon Dis. 2008;3(2):253-68. doi: 10.2147/copd.s2089. Int J Chron Obstruct Pulmon Dis. 2008. PMID: 18686734 Free PMC article. Review.
References
-
- Peto R, Chen ZM, Boreham J. Tobacco: the growing epidemic. Nat. Med. 1999;5:15–17. - PubMed
-
- Ofulue AF, Ko M. Effects of depletion of neutrophils or macrophages on development of cigarette smoke-induced emphysema. Am. J. Physiol. 1999;21:L97–L105. - PubMed
-
- Merchant RK, Schwartz DA, Helmers RA, Dayton CS, Hunninghake GW. Bronchoalveolar lavage cellularity. Am. Rev. Respir. Dis. 1992;146:448–453. - PubMed
-
- Dhami R, et al. Acute cigarette smoke-induced connective tissue breakdown is mediated by neutrophils and prevented by α1-antitrypsin. Am. J. Respir. Cell Mol. Biol. 2000;22:244–252. - PubMed
-
- Saetta M, et al. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998;157:822–826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

