The Cek1 and Hog1 mitogen-activated protein kinases play complementary roles in cell wall biogenesis and chlamydospore formation in the fungal pathogen Candida albicans
- PMID: 16467475
- PMCID: PMC1405885
- DOI: 10.1128/EC.5.2.347-358.2006
The Cek1 and Hog1 mitogen-activated protein kinases play complementary roles in cell wall biogenesis and chlamydospore formation in the fungal pathogen Candida albicans
Abstract
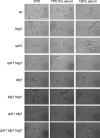
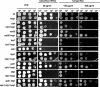
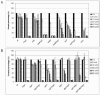
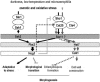
The Hog1 mitogen-activated protein (MAP) kinase mediates an adaptive response to both osmotic and oxidative stress in the fungal pathogen Candida albicans. This protein also participates in two distinct morphogenetic processes, namely the yeast-to-hypha transition (as a repressor) and chlamydospore formation (as an inducer). We show here that repression of filamentous growth occurs both under serum limitation and under other partially inducing conditions, such as low temperature, low pH, or nitrogen starvation. To understand the relationship of the HOG pathway to other MAP kinase cascades that also play a role in morphological transitions, we have constructed and characterized a set of double mutants in which we deleted both the HOG1 gene and other signaling elements (the CST20, CLA4, and HST7 kinases, the CPH1 and EFG1 transcription factors, and the CPP1 protein phosphatase). We also show that Hog1 prevents the yeast-to-hypha switch independent of all the elements analyzed and that the inability of the hog1 mutants to form chlamydospores is suppressed when additional elements of the CEK1 pathway (CST20 or HST7) are altered. Finally, we report that Hog1 represses the activation of the Cek1 MAP kinase under basal conditions and that Cek1 activation correlates with resistance to certain cell wall inhibitors (such as Congo red), demonstrating a role for this pathway in cell wall biogenesis.
Figures






Similar articles
-
Cpp1 phosphatase mediated signaling crosstalk between Hog1 and Cek1 mitogen-activated protein kinases is involved in the phenotypic transition in Candida albicans.Med Mycol. 2018 Feb 1;56(2):242-252. doi: 10.1093/mmy/myx027. Med Mycol. 2018. PMID: 28431022
-
The Sho1 adaptor protein links oxidative stress to morphogenesis and cell wall biosynthesis in the fungal pathogen Candida albicans.Mol Cell Biol. 2005 Dec;25(23):10611-27. doi: 10.1128/MCB.25.23.10611-10627.2005. Mol Cell Biol. 2005. PMID: 16287872 Free PMC article.
-
MAP Kinase Regulation of the Candida albicans Pheromone Pathway.mSphere. 2019 Feb 20;4(1):e00598-18. doi: 10.1128/mSphere.00598-18. mSphere. 2019. PMID: 30787119 Free PMC article.
-
The MAP kinase signal transduction network in Candida albicans.Microbiology (Reading). 2006 Apr;152(Pt 4):905-912. doi: 10.1099/mic.0.28616-0. Microbiology (Reading). 2006. PMID: 16549655 Review.
-
The role of MAPK signal transduction pathways in the response to oxidative stress in the fungal pathogen Candida albicans: implications in virulence.Curr Protein Pept Sci. 2010 Dec;11(8):693-703. doi: 10.2174/138920310794557655. Curr Protein Pept Sci. 2010. PMID: 21235505 Review.
Cited by
-
Candida albicans Cek1 mitogen-activated protein kinase signaling enhances fungicidal activity of salivary histatin 5.Antimicrob Agents Chemother. 2015;59(6):3460-8. doi: 10.1128/AAC.00214-15. Epub 2015 Mar 30. Antimicrob Agents Chemother. 2015. PMID: 25824232 Free PMC article.
-
The Role of Mitogen-Activated Protein (MAP) Kinase Signaling Components in the Fungal Development, Stress Response and Virulence of the Fungal Cereal Pathogen Bipolaris sorokiniana.PLoS One. 2015 May 26;10(5):e0128291. doi: 10.1371/journal.pone.0128291. eCollection 2015. PLoS One. 2015. PMID: 26011429 Free PMC article.
-
The Mkk2 MAPKK Regulates Cell Wall Biogenesis in Cooperation with the Cek1-Pathway in Candida albicans.PLoS One. 2015 Jul 21;10(7):e0133476. doi: 10.1371/journal.pone.0133476. eCollection 2015. PLoS One. 2015. PMID: 26197240 Free PMC article.
-
Small but crucial: the novel small heat shock protein Hsp21 mediates stress adaptation and virulence in Candida albicans.PLoS One. 2012;7(6):e38584. doi: 10.1371/journal.pone.0038584. Epub 2012 Jun 7. PLoS One. 2012. PMID: 22685587 Free PMC article.
-
Stress-Activated Protein Kinases in Human Fungal Pathogens.Front Cell Infect Microbiol. 2019 Jul 17;9:261. doi: 10.3389/fcimb.2019.00261. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31380304 Free PMC article. Review.
References
-
- Alonso-Monge, R., E. Real, I. Wojda, J. P. Bebelman, W. H. Mager, and M. Siderius. 2001. Hyperosmotic stress response and regulation of cell wall integrity in Saccharomyces cerevisiae share common functional aspects. Mol. Microbiol. 41:717-730. - PubMed
-
- Arana, D. M., C. Nombela, R. Alonso-Monge, and J. Pla. 2005. The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology 151:1033-1049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

