Effect of protein kinase A on accumulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) in HepG2 cell nuclei
- PMID: 16467138
- PMCID: PMC1413798
- DOI: 10.1073/pnas.0510571103
Effect of protein kinase A on accumulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) in HepG2 cell nuclei
Abstract
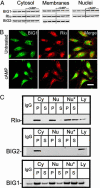
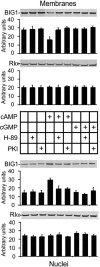
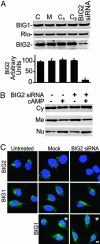
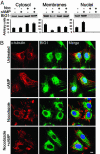
Brefeldin A-inhibited guanine nucleotide-exchange proteins, BIG1 and BIG2, are activators of ADP-ribosylation factor GTPases that are essential for regulating vesicular traffic among intracellular organelles. Biochemical analyses and immunofluorescence microscopy demonstrated BIG1 in nuclei as well as membranes and cytosol of serum-starved HepG2 cells. Within 20 min after addition of 8-Br-cAMP, BIG1 accumulated in nuclei, and this effect was blocked by protein kinase A (PKA) inhibitors H-89 and PKI, suggesting a dependence on PKA-catalyzed phosphorylation. BIG2 localization was not altered by cAMP, nor did BIG2 small interfering RNA influence nuclear accumulation of BIG1 induced by cAMP. Mutant BIG1 (S883A) in which Ala replaced Ser-883, a putative PKA phosphorylation site, did not move to the nucleus with cAMP addition, whereas replacement with Asp (S883D) resulted in nuclear accumulation of BIG1 without or with cAMP exposure, consistent with the mechanistic importance of a negative charge at that site. Mutation (712KPK714) of the nuclear localization signal inhibited BIG1 accumulation in nuclei, and PKA-catalyzed phosphorylation of S883, although necessary, was not sufficient for nuclear accumulation, as shown by the double mutation S883D/nuclear localization signal. A role for microtubules in cAMP-induced translocation of BIG1 is inferred from its inhibition by nocodazole. Thus, two more critical elements of BIG1 molecular structure were identified, as well as the potential function of microtubules in a novel PKA effect on BIG1 translocation.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Similar articles
-
Regulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) and BIG2 activity via PKA and protein phosphatase 1gamma.Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3201-6. doi: 10.1073/pnas.0611696104. Epub 2007 Feb 21. Proc Natl Acad Sci U S A. 2007. PMID: 17360629 Free PMC article.
-
Interaction of phosphodiesterase 3A with brefeldin A-inhibited guanine nucleotide-exchange proteins BIG1 and BIG2 and effect on ARF1 activity.Proc Natl Acad Sci U S A. 2009 Apr 14;106(15):6158-63. doi: 10.1073/pnas.0901558106. Epub 2009 Mar 30. Proc Natl Acad Sci U S A. 2009. PMID: 19332778 Free PMC article.
-
Enhancement of β-catenin activity by BIG1 plus BIG2 via Arf activation and cAMP signals.Proc Natl Acad Sci U S A. 2016 May 24;113(21):5946-51. doi: 10.1073/pnas.1601918113. Epub 2016 May 9. Proc Natl Acad Sci U S A. 2016. PMID: 27162341 Free PMC article.
-
BIG1 and BIG2, brefeldin A-inhibited guanine nucleotide-exchange factors for ADP-ribosylation factors.Methods Enzymol. 2005;404:174-84. doi: 10.1016/S0076-6879(05)04017-6. Methods Enzymol. 2005. PMID: 16413268
-
Regulating the regulators: role of phosphorylation in modulating the function of the GBF1/BIG family of Sec7 ARF-GEFs.FEBS Lett. 2020 Jul;594(14):2213-2226. doi: 10.1002/1873-3468.13798. Epub 2020 May 14. FEBS Lett. 2020. PMID: 32333796 Review.
Cited by
-
Effects of brefeldin A-inhibited guanine nucleotide-exchange (BIG) 1 and KANK1 proteins on cell polarity and directed migration during wound healing.Proc Natl Acad Sci U S A. 2011 Nov 29;108(48):19228-33. doi: 10.1073/pnas.1117011108. Epub 2011 Nov 14. Proc Natl Acad Sci U S A. 2011. PMID: 22084092 Free PMC article.
-
Specific functions of BIG1 and BIG2 in endomembrane organization.PLoS One. 2010 Mar 25;5(3):e9898. doi: 10.1371/journal.pone.0009898. PLoS One. 2010. PMID: 20360857 Free PMC article.
-
Novel C-terminal motif within Sec7 domain of guanine nucleotide exchange factors regulates ADP-ribosylation factor (ARF) binding and activation.J Biol Chem. 2011 Oct 21;286(42):36898-906. doi: 10.1074/jbc.M111.230631. Epub 2011 Aug 2. J Biol Chem. 2011. PMID: 21828055 Free PMC article.
-
Podosome assembly is controlled by the GTPase ARF1 and its nucleotide exchange factor ARNO.J Cell Biol. 2017 Jan 2;216(1):181-197. doi: 10.1083/jcb.201605104. Epub 2016 Dec 22. J Cell Biol. 2017. PMID: 28007915 Free PMC article.
-
Regulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) and BIG2 activity via PKA and protein phosphatase 1gamma.Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3201-6. doi: 10.1073/pnas.0611696104. Epub 2007 Feb 21. Proc Natl Acad Sci U S A. 2007. PMID: 17360629 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

