A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors
- PMID: 16451849
- PMCID: PMC1367826
- DOI: 10.1289/ehp.8284
A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors
Abstract
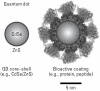
As a growing applied science, nanotechnology has considerable global socioeconomic value, and the benefits afforded by nanoscale materials and processes are expected to have significant impacts on almost all industries and all areas of society. A diverse array of engineered nanoscale products and processes have emerged [e.g., carbon nanotubes, fullerene derivatives, and quantum dots (QDs)], with widespread applications in fields such as medicine, plastics, energy, electronics, and aerospace. With the nanotechnology economy estimated to be valued at dollar 1 trillion by 2012, the prevalence of these materials in society will be increasing, as will the likelihood of exposures. Importantly, the vastness and novelty of the nanotechnology frontier leave many areas unexplored, or underexplored, such as the potential adverse human health effects resulting from exposure to novel nanomaterials. It is within this context that the need for understanding the potentially harmful side effects of these materials becomes clear. The reviewed literature suggests several key points: Not all QDs are alike; engineered QDs cannot be considered a uniform group of substances. QD absorption, distribution, metabolism, excretion, and toxicity depend on multiple factors derived from both inherent physicochemical properties and environmental conditions; QD size, charge, concentration, outer coating bioactivity (capping material and functional groups), and oxidative, photolytic, and mechanical stability have each been implicated as determining factors in QD toxicity. Although they offer potentially invaluable societal benefits such as drug targeting and in vivo biomedical imaging, QDs may also pose risks to human health and the environment under certain conditions. Key words: environment, human health, nanomaterials, nanosized particles, nanotechnology, nanotoxicology, quantum dots, toxicology.
Figures
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Australia in 2030: what is our path to health for all?Med J Aust. 2021 May;214 Suppl 8:S5-S40. doi: 10.5694/mja2.51020. Med J Aust. 2021. PMID: 33934362
-
Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article.
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article. Review.
Cited by
-
Quantum Dots as a Potential Multifunctional Material for the Enhancement of Clinical Diagnosis Strategies and Cancer Treatments.Nanomaterials (Basel). 2024 Jun 25;14(13):1088. doi: 10.3390/nano14131088. Nanomaterials (Basel). 2024. PMID: 38998693 Free PMC article. Review.
-
Nanomaterial interactions with and trafficking across the lung alveolar epithelial barrier: implications for health effects of air-pollution particles.Air Qual Atmos Health. 2011 Mar 1;4(1):65-78. doi: 10.1007/s11869-010-0098-z. Air Qual Atmos Health. 2011. PMID: 25568662 Free PMC article.
-
Biocompatible Magnetic Conjugated Polymer Nanoparticles for Optical and Lifetime Imaging Applications in the First Biological Window.ACS Appl Polym Mater. 2022 Nov 11;4(11):8193-8202. doi: 10.1021/acsapm.2c01153. Epub 2022 Oct 12. ACS Appl Polym Mater. 2022. PMID: 36405304 Free PMC article.
-
Bimodal Imaging Using Neodymium Doped Gadolinium Fluoride Nanocrystals with Near-Infrared to Near-Infrared Downconversion Luminescence and Magnetic Resonance Properties.J Mater Chem B. 2013 Nov 7;1(41):5702-5710. doi: 10.1039/C3TB20905A. J Mater Chem B. 2013. PMID: 25584192 Free PMC article.
-
Facile green synthesis of silicon nanoparticles from Equisetum arvense for fluorescence based detection of Fe(iii) ions.Nanoscale Adv. 2020 Aug 4;2(9):4125-4132. doi: 10.1039/d0na00307g. eCollection 2020 Sep 16. Nanoscale Adv. 2020. PMID: 36132780 Free PMC article.
References
-
- Aldana J, Wang YA, Peng X. Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J Am Chem Soc. 2001;123:8844–8850. - PubMed
-
- Alivisatos P. The use of nanocrystals in biological detection. Nat Biotechnol. 2004;22:47–52. - PubMed
-
- Ballou B, Lagerholm BC, Ernst LA, Bruchez MP, Waggoner AS. Noninvasive imaging of quantum dots in mice. Bioconjug Chem. 2004;15(1):79–86. - PubMed
-
- Beaurepaire E, Buissette V, Sauviat MP, Giaume D, Lahlil K, Mercuri A, et al. Functionalized fluorescent oxide nanoparticles: artificial toxins for sodium channel targeting and imaging at the single-molecule level. Nano Lett. 2004;4(11):2079–2083.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials