Presenilin-1 uses phospholipase D1 as a negative regulator of beta-amyloid formation
- PMID: 16449386
- PMCID: PMC1413665
- DOI: 10.1073/pnas.0510708103
Presenilin-1 uses phospholipase D1 as a negative regulator of beta-amyloid formation
Abstract
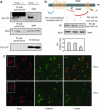
Presenilin (PS1/PS2) is a major component of gamma-secretase, the activity that mediates proteolysis of beta-amyloid precursor protein to generate beta-amyloid (Abeta). Here we demonstrate that PS1, through its loop region, binds to phospholipase D1 (PLD1), thereby recruiting it to the Golgi/trans-Golgi network. Overexpression of wild-type PLD1 reduces Abeta generation. Conversely, down-regulation of endogenous PLD1 by small hairpin RNA elevates Abeta production. The Abeta-lowering effect of PLD1 is independent of its ability to promote vesicular budding of beta-amyloid precursor protein. The data indicate that overexpression of PLD1 decreases, and down-regulation of PLD1 increases, the catalytic activity, and the association of the subunits, of gamma-secretase.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

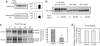


Similar articles
-
Intracellular trafficking of presenilin 1 is regulated by beta-amyloid precursor protein and phospholipase D1.J Biol Chem. 2009 May 1;284(18):12145-52. doi: 10.1074/jbc.M808497200. Epub 2009 Mar 10. J Biol Chem. 2009. PMID: 19276086 Free PMC article.
-
Phospholipase D1 corrects impaired betaAPP trafficking and neurite outgrowth in familial Alzheimer's disease-linked presenilin-1 mutant neurons.Proc Natl Acad Sci U S A. 2006 Feb 7;103(6):1936-40. doi: 10.1073/pnas.0510710103. Epub 2006 Jan 31. Proc Natl Acad Sci U S A. 2006. PMID: 16449385 Free PMC article.
-
Failure of the interaction between presenilin 1 and the substrate of gamma-secretase to produce Abeta in insect cells.J Neurochem. 2002 Oct;83(2):390-9. doi: 10.1046/j.1471-4159.2002.01138.x. J Neurochem. 2002. PMID: 12423249
-
Abeta-generating enzymes: recent advances in beta- and gamma-secretase research.Neuron. 2000 Sep;27(3):419-22. doi: 10.1016/s0896-6273(00)00051-9. Neuron. 2000. PMID: 11055423 Review. No abstract available.
-
Distinct presenilin-dependent and presenilin-independent gamma-secretases are responsible for total cellular Abeta production.J Neurosci Res. 2003 Nov 1;74(3):361-9. doi: 10.1002/jnr.10776. J Neurosci Res. 2003. PMID: 14598312 Review.
Cited by
-
Reduction in CHT1-mediated choline uptake in primary neurons from presenilin-1 M146V mutant knock-in mice.Brain Res. 2007 Mar 2;1135(1):12-21. doi: 10.1016/j.brainres.2006.12.005. Epub 2006 Dec 29. Brain Res. 2007. PMID: 17196556 Free PMC article.
-
Neuronal protein trafficking associated with Alzheimer disease: from APP and BACE1 to glutamate receptors.Cell Adh Migr. 2009 Jan-Mar;3(1):118-28. doi: 10.4161/cam.3.1.7254. Epub 2009 Jan 21. Cell Adh Migr. 2009. PMID: 19372755 Free PMC article. Review.
-
Trafficking regulation of proteins in Alzheimer's disease.Mol Neurodegener. 2014 Jan 11;9:6. doi: 10.1186/1750-1326-9-6. Mol Neurodegener. 2014. PMID: 24410826 Free PMC article. Review.
-
Presenilin-2 and Calcium Handling: Molecules, Organelles, Cells and Brain Networks.Cells. 2020 Sep 25;9(10):2166. doi: 10.3390/cells9102166. Cells. 2020. PMID: 32992716 Free PMC article. Review.
-
The heme degradation pathway is a promising serum biomarker source for the early detection of Alzheimer's disease.J Alzheimers Dis. 2010;19(3):1081-91. doi: 10.3233/JAD-2010-1303. J Alzheimers Dis. 2010. PMID: 20157261 Free PMC article.
References
-
- Haass C., De Strooper B. Science. 1999;286:916–919. - PubMed
-
- De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., Von Figura K., Van Leuven F. Nature. 1998;391:387–390. - PubMed
-
- Borchelt D. R., Thinakaran G., Eckman C. B., Lee M. K., Davenport F., Ratovitsky T., Prada C. M., Kim G., Seekins S., Yager D., et al. Neuron. 1996;17:1005–1013. - PubMed
-
- Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W., et al. Nat. Med. 1996;2:864–870. - PubMed
-
- Sisodia S. S., St George-Hyslop P. H. Nat. Rev. Neurosci. 2002;3:281–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

