Stable silencing of SNAP-25 in PC12 cells by RNA interference
- PMID: 16445859
- PMCID: PMC1373637
- DOI: 10.1186/1471-2202-7-9
Stable silencing of SNAP-25 in PC12 cells by RNA interference
Abstract
Background: SNAP-25 is a synaptic protein known to be involved in exocytosis of synaptic vesicles in neurons and of large dense-core vesicles in neuroendocrine cells. Its role in exocytosis has been studied in SNAP-25 knockout mice, in lysed synaptosomes lacking functional SNAP-25 and in cells after treatment with botulinum toxins A or E that specifically cleave SNAP-25. These studies have shown that SNAP-25 appears to be required for most but not all evoked secretion. In order to further study the role of SNAP-25 in catecholamine secretion from PC12 cells we have used the recently developed technique of RNA interference to generate PC12 cell lines with virtually undetectable levels of SNAP-25. RNA interference is the sequence-specific silencing or knockdown of gene expression triggered by the introduction of double-stranded RNA into a cell. RNA interference can be elicited in mammalian cells in a number of ways, one of which is by the expression of small hairpin RNAs from a transfected plasmid. Selection of stably transfected cell lines expressing a small hairpin RNA allows one-time characterization of the degree and specificity of gene silencing and affords a continuing source of well-characterized knockdown cells for experimentation.
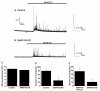
Results: A PC12 cell line stably transfected with a plasmid expressing an shRNA targeting SNAP-25 has been established. This SNAP-25 knockdown cell line has barely detectable levels of SNAP-25, but normal levels of other synaptic proteins. Catecholamine secretion elicited by depolarization of the SNAP-25 knockdown cells was reduced to 37% of control.
Conclusion: Knockdown of SNAP-25 in PC12 cells reduces but does not eliminate evoked secretion of catecholamines. Transient expression of human SNAP-25 in the knockdown cells rescues the deficit in catecholamine secretion.
Figures

Similar articles
-
The C2B domain of rabphilin directly interacts with SNAP-25 and regulates the docking step of dense core vesicle exocytosis in PC12 cells.J Biol Chem. 2005 Nov 25;280(47):39253-9. doi: 10.1074/jbc.M507173200. Epub 2005 Oct 3. J Biol Chem. 2005. PMID: 16203731
-
Variability in RNA interference in neuroendocrine PC12 cell lines stably transfected with an shRNA plasmid.J Neurosci Methods. 2007 Nov 30;166(2):236-40. doi: 10.1016/j.jneumeth.2007.07.016. Epub 2007 Aug 1. J Neurosci Methods. 2007. PMID: 17767962
-
Importance of two adjacent C-terminal sequences of SNAP-25 in exocytosis from intact and permeabilized chromaffin cells revealed by inhibition with botulinum neurotoxins A and E.Biochemistry. 1997 Mar 18;36(11):3061-7. doi: 10.1021/bi9622478. Biochemistry. 1997. PMID: 9115981
-
Interaction of anesthetics with neurotransmitter release machinery proteins.J Neurophysiol. 2013 Feb;109(3):758-67. doi: 10.1152/jn.00666.2012. Epub 2012 Nov 7. J Neurophysiol. 2013. PMID: 23136341 Free PMC article.
-
Molecular analysis of SNAP-25 function in exocytosis.Ann N Y Acad Sci. 2002 Oct;971:210-21. doi: 10.1111/j.1749-6632.2002.tb04465.x. Ann N Y Acad Sci. 2002. PMID: 12438121 Review.
Cited by
-
Differential roles of Trk and p75 neurotrophin receptors in tumorigenesis and chemoresistance ex vivo and in vivo.Cancer Chemother Pharmacol. 2010 May;65(6):1047-56. doi: 10.1007/s00280-009-1110-x. Epub 2009 Aug 22. Cancer Chemother Pharmacol. 2010. PMID: 19701634 Free PMC article.
-
A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking.Nat Neurosci. 2010 Mar;13(3):338-43. doi: 10.1038/nn.2488. Epub 2010 Jan 31. Nat Neurosci. 2010. PMID: 20118925 Free PMC article.
-
Microarray-based analysis of cell-cycle gene expression during spermatogenesis in the mouse.Biol Reprod. 2010 Oct;83(4):663-75. doi: 10.1095/biolreprod.110.084889. Epub 2010 Jul 14. Biol Reprod. 2010. PMID: 20631398 Free PMC article.
-
Mitochondria-Endoplasmic Reticulum Interplay Regulates Exo-Cytosis in Human Neuroblastoma Cells.Cells. 2022 Feb 2;11(3):514. doi: 10.3390/cells11030514. Cells. 2022. PMID: 35159324 Free PMC article.
-
ERK and PDE4 cooperate to induce RAF isoform switching in melanoma.Nat Struct Mol Biol. 2011 May;18(5):584-91. doi: 10.1038/nsmb.2022. Epub 2011 Apr 10. Nat Struct Mol Biol. 2011. PMID: 21478863
References
-
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, Molnar Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

