Laminin-5 (laminin-332): Unique biological activity and role in tumor growth and invasion
- PMID: 16441418
- PMCID: PMC11159065
- DOI: 10.1111/j.1349-7006.2006.00150.x
Laminin-5 (laminin-332): Unique biological activity and role in tumor growth and invasion
Abstract
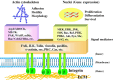
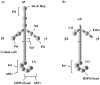
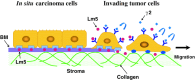
The development and progression of tumor cells is controlled by their interactions with neighboring host cells and a variety of microenvironmental factors including extracellular matrix (ECM) molecules, growth factors and proteinases. Cell-adhesive ECM proteins are a prerequisite for growth and migration of many types of cells. Their interactions with integrins and other cell surface receptors induce intracellular signaling that regulates the actin cytoskeleton and gene expression. The basement membrane protein laminin-5 is a notable cell adhesion molecule, which promotes cellular adhesion and migration much more efficiently than other ECM proteins. There is accumulating evidence that laminin-5 is involved in tumor growth and progression. With special reference to laminin-5, this article reviews the regulatory mechanisms of cellular adhesion and migration by ECM molecules and their significance in tumor progression.
Figures



Similar articles
-
The lutheran/basal cell adhesion molecule promotes tumor cell migration by modulating integrin-mediated cell attachment to laminin-511 protein.J Biol Chem. 2013 Oct 25;288(43):30990-1001. doi: 10.1074/jbc.M113.486456. Epub 2013 Sep 13. J Biol Chem. 2013. PMID: 24036115 Free PMC article.
-
The role of cell adhesion proteins--laminin and fibronectin--in the movement of malignant and metastatic cells.Cancer Metastasis Rev. 1985;4(2):125-52. doi: 10.1007/BF00050692. Cancer Metastasis Rev. 1985. PMID: 3893683 Review.
-
The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells.Rep Prog Phys. 2019 Jun;82(6):064602. doi: 10.1088/1361-6633/ab1628. Epub 2019 Apr 4. Rep Prog Phys. 2019. PMID: 30947151 Review.
-
Laminin-induced signaling in tumor cells.Cancer Lett. 2005 Jun 1;223(1):1-10. doi: 10.1016/j.canlet.2004.08.030. Cancer Lett. 2005. PMID: 15890231 Review.
-
The opposing roles of laminin-binding integrins in cancer.Matrix Biol. 2017 Jan;57-58:213-243. doi: 10.1016/j.matbio.2016.08.007. Epub 2016 Aug 22. Matrix Biol. 2017. PMID: 27562932 Review.
Cited by
-
Overexpression of Laminin 5γ2 Chain Correlates with Tumor Cell Proliferation, Invasion, and Poor Prognosis in Laryngeal Squamous Cell Carcinoma.J Oncol. 2022 Oct 15;2022:7248064. doi: 10.1155/2022/7248064. eCollection 2022. J Oncol. 2022. PMID: 36284634 Free PMC article.
-
Potentiation of tumor cell invasion by co-culture with monocytes accompanying enhanced production of matrix metalloproteinase and fibronectin.Clin Exp Metastasis. 2013 Mar;30(3):289-97. doi: 10.1007/s10585-012-9536-7. Epub 2012 Sep 29. Clin Exp Metastasis. 2013. PMID: 23053742
-
Multi-omics profiling reveals key signaling pathways in ovarian cancer controlled by STAT3.Theranostics. 2019 Jul 28;9(19):5478-5496. doi: 10.7150/thno.33444. eCollection 2019. Theranostics. 2019. PMID: 31534498 Free PMC article.
-
Tumor budding index and microvessel density assessment in patients with endometrial cancer: A pilot study.Oncol Lett. 2020 Sep;20(3):2701-2710. doi: 10.3892/ol.2020.11811. Epub 2020 Jul 3. Oncol Lett. 2020. PMID: 32782586 Free PMC article.
-
Assessment of the tumourigenic and metastatic properties of SK-MEL28 melanoma cells surviving electrochemotherapy with bleomycin.Radiol Oncol. 2012 Mar;46(1):32-45. doi: 10.2478/v10019-012-0010-6. Epub 2012 Jan 12. Radiol Oncol. 2012. PMID: 22933978 Free PMC article.
References
-
- Liotta LA, Kohn EC. The microenvironment of the tumour–host interface. Nature 2001; 411: 375–9. - PubMed
-
- Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 2000; 18: 1135–49. - PubMed
-
- Liotta LA. Tumor invasion and metastases − role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res 1986; 46: 1–7. - PubMed
-
- Sato H, Takino T, Okada Y et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994; 370: 61–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

