Rapid activation of ATR by ionizing radiation requires ATM and Mre11
- PMID: 16431910
- PMCID: PMC1821075
- DOI: 10.1074/jbc.M513265200
Rapid activation of ATR by ionizing radiation requires ATM and Mre11
Abstract
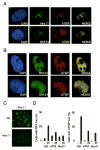
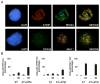
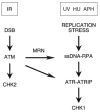
The ataxia-telangiectasia-mutated (ATM) and ATM- and Rad3-related (ATR) protein kinases are crucial regulatory proteins in genotoxic stress response pathways that pause the cell cycle to permit DNA repair. Here we show that Chk1 phosphorylation in response to hydroxyurea and ultraviolet radiation is ATR-dependent and ATM- and Mre11-independent. In contrast, Chk1 phosphorylation in response to ionizing radiation (IR) is dependent on ATR, ATM, and Mre11. The ATR and ATM/Mre11 pathways are generally thought to be separate with ATM activation occurring early and ATR activation occurring as a late response to double strand breaks. However, we demonstrate that ATR is activated rapidly by IR, and ATM and Mre11 enhance ATR signaling. ATR-ATR-interacting protein recruitment to double strand breaks is less efficient in the absence of ATM and Mre11. Furthermore, IR-induced replication protein A foci formation is defective in ATM- and Mre11-deficient cells. Thus, ATM and Mre11 may stimulate the ATR signaling pathway by converting DNA damage generated by IR into structures that recruit and activate ATR.
Figures


Similar articles
-
ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks.Nat Cell Biol. 2006 Jan;8(1):37-45. doi: 10.1038/ncb1337. Epub 2005 Dec 4. Nat Cell Biol. 2006. PMID: 16327781
-
ATM regulates ATR chromatin loading in response to DNA double-strand breaks.J Exp Med. 2006 Feb 20;203(2):297-303. doi: 10.1084/jem.20051923. Epub 2006 Feb 6. J Exp Med. 2006. PMID: 16461339 Free PMC article.
-
Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation.J Biol Chem. 2003 Apr 25;278(17):14806-11. doi: 10.1074/jbc.M210862200. Epub 2003 Feb 14. J Biol Chem. 2003. PMID: 12588868
-
The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer.Adv Cancer Res. 2010;108:73-112. doi: 10.1016/B978-0-12-380888-2.00003-0. Adv Cancer Res. 2010. PMID: 21034966 Review.
-
Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis.DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1219-25. doi: 10.1016/j.dnarep.2004.04.009. DNA Repair (Amst). 2004. PMID: 15279810 Review.
Cited by
-
Intrinsic ATR signaling shapes DNA end resection and suppresses toxic DNA-PKcs signaling.NAR Cancer. 2020 Jun;2(2):zcaa006. doi: 10.1093/narcan/zcaa006. Epub 2020 May 1. NAR Cancer. 2020. PMID: 32743550 Free PMC article.
-
"ATR activation in response to ionizing radiation: still ATM territory".Cell Div. 2006 May 17;1(1):7. doi: 10.1186/1747-1028-1-7. Cell Div. 2006. PMID: 16759429 Free PMC article.
-
Distinct roles of the ATR kinase and the Mre11-Rad50-Nbs1 complex in the maintenance of chromosomal stability in Arabidopsis.Plant Cell. 2010 Sep;22(9):3020-33. doi: 10.1105/tpc.110.078527. Epub 2010 Sep 28. Plant Cell. 2010. PMID: 20876831 Free PMC article.
-
Human CtIP promotes DNA end resection.Nature. 2007 Nov 22;450(7169):509-14. doi: 10.1038/nature06337. Epub 2007 Oct 28. Nature. 2007. PMID: 17965729 Free PMC article.
-
DNA damage response at functional and dysfunctional telomeres.Genes Dev. 2008 Jan 15;22(2):125-40. doi: 10.1101/gad.1626908. Genes Dev. 2008. PMID: 18198332 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

