Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers
- PMID: 16401722
- PMCID: PMC2063555
- DOI: 10.1083/jcb.200506179
Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers
Abstract
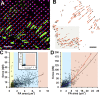
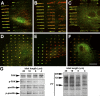
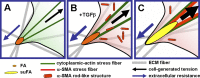
Expression of alpha-smooth muscle actin (alpha-SMA) renders fibroblasts highly contractile and hallmarks myofibroblast differentiation. We identify alpha-SMA as a mechanosensitive protein that is recruited to stress fibers under high tension. Generation of this threshold tension requires the anchoring of stress fibers at sites of 8-30-microm-long "supermature" focal adhesions (suFAs), which exert a stress approximately fourfold higher (approximately 12 nN/microm2) on micropatterned deformable substrates than 2-6-microm-long classical FAs. Inhibition of suFA formation by growing myofibroblasts on substrates with a compliance of < or = 11 kPa and on rigid micropatterns of 6-microm-long classical FA islets confines alpha-SMA to the cytosol. Reincorporation of alpha-SMA into stress fibers is established by stretching 6-microm-long classical FAs to 8.1-microm-long suFA islets on extendable membranes; the same stretch producing 5.4-microm-long classical FAs from initially 4-microm-long islets is without effect. We propose that the different molecular composition and higher phosphorylation of FAs on supermature islets, compared with FAs on classical islets, accounts for higher stress resistance.
Figures
Similar articles
-
Rho-mediated assembly of stress fibers is differentially regulated in corneal fibroblasts and myofibroblasts.Exp Cell Res. 2004 Aug 15;298(2):574-83. doi: 10.1016/j.yexcr.2004.05.005. Exp Cell Res. 2004. PMID: 15265703
-
[Effect of actomyosin contractility on focal contacts of myofibroblasts and structure of stress fibers].Tsitologiia. 2002;44(1):48-55. Tsitologiia. 2002. PMID: 11868461 Russian.
-
Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts.Mol Biol Cell. 2003 Jun;14(6):2508-19. doi: 10.1091/mbc.e02-11-0729. Epub 2003 Feb 21. Mol Biol Cell. 2003. PMID: 12808047 Free PMC article.
-
Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission.Eur J Cell Biol. 2006 Apr;85(3-4):175-81. doi: 10.1016/j.ejcb.2005.09.004. Epub 2005 Oct 10. Eur J Cell Biol. 2006. PMID: 16546559 Review.
-
Mechanisms of force generation and transmission by myofibroblasts.Curr Opin Biotechnol. 2003 Oct;14(5):538-46. doi: 10.1016/j.copbio.2003.08.006. Curr Opin Biotechnol. 2003. PMID: 14580586 Review.
Cited by
-
A TRP to cardiac fibroblast differentiation.Channels (Austin). 2013 May-Jun;7(3):211-4. doi: 10.4161/chan.24328. Epub 2013 Mar 19. Channels (Austin). 2013. PMID: 23511028 Free PMC article.
-
The constant beat: cardiomyocytes adapt their forces by equal contraction upon environmental stiffening.Biol Open. 2013 Mar 15;2(3):351-61. doi: 10.1242/bio.20133830. Epub 2013 Jan 30. Biol Open. 2013. PMID: 23519595 Free PMC article.
-
Tacrolimus Modulates TGF-β Signaling to Induce Epithelial-Mesenchymal Transition in Human Renal Proximal Tubule Epithelial Cells.J Clin Med. 2016 Apr 26;5(5):50. doi: 10.3390/jcm5050050. J Clin Med. 2016. PMID: 27128949 Free PMC article.
-
The myofibroblast in wound healing and fibrosis: answered and unanswered questions.F1000Res. 2016 Apr 26;5:F1000 Faculty Rev-752. doi: 10.12688/f1000research.8190.1. eCollection 2016. F1000Res. 2016. PMID: 27158462 Free PMC article. Review.
-
Actin depolymerization under force is governed by lysine 113:glutamic acid 195-mediated catch-slip bonds.Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):5022-7. doi: 10.1073/pnas.1218407110. Epub 2013 Mar 4. Proc Natl Acad Sci U S A. 2013. PMID: 23460697 Free PMC article.
References
-
- Balaban, N.Q., U.S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, and B. Geiger. 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–472. - PubMed
-
- Bao, G., and S. Suresh. 2003. Cell and molecular mechanics of biological materials. Nat. Mater. 2:715–725. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

