Estrogen-like properties of fluorotelomer alcohols as revealed by mcf-7 breast cancer cell proliferation
- PMID: 16393665
- PMCID: PMC1332663
- DOI: 10.1289/ehp.8149
Estrogen-like properties of fluorotelomer alcohols as revealed by mcf-7 breast cancer cell proliferation
Abstract
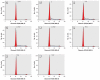
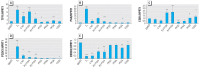
We investigated estrogen-like properties of five perfluorinated compounds using a combination of three in vitro assays. By means of an E-screen assay, we detected the proliferation-promoting capacity of the fluorotelomer alcohols 1H,1H,2H,2H-perfluorooctan-1-ol (6:2 FTOH) and 1H,1H,2H,2H-perfluoro-decan-1-ol (8:2 FTOH). The more widely environmentally distributed compounds perfluoro-1-octane sulfonate, perfluorooctanoic acid, and perfluorononanoic acid did not seem to possess this hormone-dependent proliferation capacity. We investigated cell cycle dynamics using flow cytometric analyses of the DNA content of the nuclei of MCF-7 breast cancer cells. Exposure to both fluorotelomer alcohols stimulated resting MCF-7 cells to reenter the synthesis phase (S-phase) of the cell cycle. After only 24 hr of treatment, we observed significant increases in the percentage of cells in the S-phase. In order to further investigate the resemblance of the newly detected xenoestrogens to the reference compound 17beta-estradiol (E2), gene expression of a number of estrogen-responsive genes was analyzed by real-time polymerase chain reaction. With E2, as well as 4-nonylphenol and the fluorotelomer alcohols, we observed up-regulation of trefoil factor 1, progesterone receptor, and PDZK1 and down-regulation of ERBB2 gene expression. We observed small but relevant up-regulation of the estrogen receptor as a consequence of exposures to 6:2 FTOH or 8:2 FTOH. The latter finding suggests an alternative mode of action of the fluorotelomer alcohols compared with that of E2. This study clearly underlines the need for future in vivo testing for specific endocrine-related end points.
Figures

Similar articles
-
Fluorotelomer alcohols induce hepatic vitellogenin through activation of the estrogen receptor in male medaka (Oryzias latipes).Chemosphere. 2008 May;71(10):1853-9. doi: 10.1016/j.chemosphere.2008.01.065. Epub 2008 Mar 10. Chemosphere. 2008. PMID: 18334264
-
Estrogenic effects of fluorotelomer alcohols for human estrogen receptor isoforms alpha and beta in vitro.Biol Pharm Bull. 2007 Jul;30(7):1358-9. doi: 10.1248/bpb.30.1358. Biol Pharm Bull. 2007. PMID: 17603182
-
Flow cytometric cell cycle analysis allows for rapid screening of estrogenicity in MCF-7 breast cancer cells.Toxicol In Vitro. 2006 Oct;20(7):1238-48. doi: 10.1016/j.tiv.2006.05.002. Epub 2006 May 16. Toxicol In Vitro. 2006. PMID: 16797915
-
Endocrine therapy resistance can be associated with high estrogen receptor alpha (ERalpha) expression and reduced ERalpha phosphorylation in breast cancer models.Endocr Relat Cancer. 2006 Dec;13(4):1121-33. doi: 10.1677/erc.1.01257. Endocr Relat Cancer. 2006. PMID: 17158758
-
Comparative analysis of the toxicological databases for 6:2 fluorotelomer alcohol (6:2 FTOH) and perfluorohexanoic acid (PFHxA).Food Chem Toxicol. 2020 Apr;138:111210. doi: 10.1016/j.fct.2020.111210. Epub 2020 Feb 19. Food Chem Toxicol. 2020. PMID: 32087313 Review.
Cited by
-
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary.Hum Reprod Update. 2020 Sep 1;26(5):724-752. doi: 10.1093/humupd/dmaa018. Hum Reprod Update. 2020. PMID: 32476019 Free PMC article.
-
Promotion of hepatocarcinogenesis by perfluoroalkyl acids in rainbow trout.Toxicol Sci. 2012 Jan;125(1):69-78. doi: 10.1093/toxsci/kfr267. Epub 2011 Oct 9. Toxicol Sci. 2012. PMID: 21984479 Free PMC article.
-
PPARα-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling.Toxicology. 2017 Jul 15;387:95-107. doi: 10.1016/j.tox.2017.05.013. Epub 2017 May 27. Toxicology. 2017. PMID: 28558994 Free PMC article.
-
A toxicogenomics-based identification of potential mechanisms and signaling pathways involved in PFCs-induced cancer in human.Toxicol Res (Camb). 2024 Sep 24;13(5):tfae151. doi: 10.1093/toxres/tfae151. eCollection 2024 Oct. Toxicol Res (Camb). 2024. PMID: 39323479 Review.
-
Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro.Toxicol Sci. 2011 Mar;120(1):42-58. doi: 10.1093/toxsci/kfq379. Epub 2010 Dec 16. Toxicol Sci. 2011. PMID: 21163906 Free PMC article.
References
-
- Bièche I, Parfait B, Le Doussal V, Olivi M, Rio M, Lidereau R, et al. Identification of CGA as a novel estrogen receptor-responsive gene in breast cancer: an outstanding candidate marker to predict the response to endocrine therapy. Cancer Res. 2001;61:1652–1658. - PubMed
-
- Degen GH, Bolt HM. Endocrine disruptors: update on xenoestrogens. Int Arch Occup Environ Health. 2000;73:433–441. - PubMed
-
- Dimitrov S, Kamenska V, Walker JD, Windle W, Purdy R, Lewis M, et al. Predicting the biodegradation products of perfluorinated chemicals using CATABOL. SAR QSAR Environ Res. 2004;15:69–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

