Involvement of template-activating factor I/SET in transcription of adenovirus early genes as a positive-acting factor
- PMID: 16378981
- PMCID: PMC1346848
- DOI: 10.1128/JVI.80.2.794-801.2006
Involvement of template-activating factor I/SET in transcription of adenovirus early genes as a positive-acting factor
Abstract
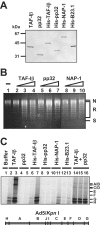
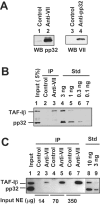
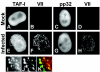
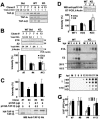
The adenovirus genome complexed with viral core protein VII (adenovirus DNA-protein VII complex) at least is the bona fide template for transcription of adenovirus early genes. It is believed that the highly basic protein VII, like cellular histones, is a negative regulator for genome functions. Analyses with in vitro replication and transcription systems using the adenovirus DNA-protein VII complex have revealed that remodeling of the complex is crucial for efficient DNA replication and transcription. We identified host acidic proteins, template-activating factor I (TAF-I), TAF-II, and TAF-III as stimulatory factors for replication from the adenovirus DNA-protein VII complex. Recently, it was reported that the adenovirus DNA interacts with TAF-I and pp32, another host acidic protein (Y. Xue, J. S. Johnson, D. A. Ornelles, J. Lieberman, and D. A. Engel, J. Virol. 79:2474-2483, 2005). We found that TAF-I interacts and colocalizes with protein VII in adenovirus-infected cells during the early phases of infection, but pp32 does not. Although pp32 had the potential ability to interact with protein VII, pp32 did not remodel the adenovirus DNA-protein VII complex in vitro. Small interfering RNA-mediated knockdown of TAF-I expression leads to the delay of the transcription timing of early genes. These results provide evidence that TAF-I plays an important role in the early stages of the adenovirus infection cycle.
Figures
Similar articles
-
Binding modes of the precursor of adenovirus major core protein VII to DNA and template activating factor I: implication for the mechanism of remodeling of the adenovirus chromatin.Biochemistry. 2006 Jan 10;45(1):303-13. doi: 10.1021/bi051248+. Biochemistry. 2006. PMID: 16388607
-
Ternary complex formation between DNA-adenovirus core protein VII and TAF-Ibeta/SET, an acidic molecular chaperone.FEBS Lett. 2003 Dec 18;555(3):521-7. doi: 10.1016/s0014-5793(03)01336-x. FEBS Lett. 2003. PMID: 14675767
-
Coiled-coil structure-mediated dimerization of template activating factor-I is critical for its chromatin remodeling activity.J Mol Biol. 1999 Jul 9;290(2):547-57. doi: 10.1006/jmbi.1999.2898. J Mol Biol. 1999. PMID: 10390352
-
Chromatin structure of adenovirus DNA throughout infection.Nucleic Acids Res. 2012 Mar;40(6):2369-76. doi: 10.1093/nar/gkr1076. Epub 2011 Nov 23. Nucleic Acids Res. 2012. PMID: 22116065 Free PMC article. Review.
-
TAF(II)250: a transcription toolbox.J Cell Sci. 2001 Aug;114(Pt 16):2895-902. doi: 10.1242/jcs.114.16.2895. J Cell Sci. 2001. PMID: 11686293 Review.
Cited by
-
DNA replication-dependent binding of CTCF plays a critical role in adenovirus genome functions.Sci Rep. 2013;3:2187. doi: 10.1038/srep02187. Sci Rep. 2013. PMID: 23851926 Free PMC article.
-
Adenovirus Core Protein VII Downregulates the DNA Damage Response on the Host Genome.J Virol. 2017 Sep 27;91(20):e01089-17. doi: 10.1128/JVI.01089-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28794020 Free PMC article.
-
PP2A complex disruptor SET prompts widespread hypertranscription of growth-essential genes in the pancreatic cancer cells.Sci Adv. 2024 Jan 26;10(4):eadk6633. doi: 10.1126/sciadv.adk6633. Epub 2024 Jan 26. Sci Adv. 2024. PMID: 38277454 Free PMC article.
-
Assembly and remodeling of viral DNA and RNA replicons regulated by cellular molecular chaperones.Biophys Rev. 2018 Apr;10(2):445-452. doi: 10.1007/s12551-017-0333-z. Epub 2017 Nov 22. Biophys Rev. 2018. PMID: 29170971 Free PMC article. Review.
-
Promoter region-specific histone incorporation by the novel histone chaperone ANP32B and DNA-binding factor KLF5.Mol Cell Biol. 2008 Feb;28(3):1171-81. doi: 10.1128/MCB.01396-07. Epub 2007 Nov 26. Mol Cell Biol. 2008. PMID: 18039846 Free PMC article.
References
-
- Anderson, C. W., M. E. Young, and S. J. Flint. 1989. Characterization of the adenovirus 2 virion protein, mu. Virology 172:506-512. - PubMed
-
- Beresford, P. J., D. Zhang, D. Y. Oh, Z. Fan, E. L. Greer, M. L. Russo, M. Jaju, and J. Lieberman. 2001. Granzyme A activates an endoplasmic reticulum-associated caspase-independent nuclease to induce single-stranded DNA nicks. J. Biol. Chem. 276:43285-43293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

