Resident pleural macrophages are key orchestrators of neutrophil recruitment in pleural inflammation
- PMID: 16357332
- PMCID: PMC2662938
- DOI: 10.1164/rccm.200504-538OC
Resident pleural macrophages are key orchestrators of neutrophil recruitment in pleural inflammation
Abstract
Rationale: The role played by resident pleural macrophages in the initiation of pleural inflammation is currently unclear.
Objective: To evaluate the role of resident pleural macrophages in the initiation of inflammation.
Methods: We have used a conditional macrophage ablation strategy to determine the role of resident pleural macrophages in the regulation of neutrophil recruitment in a murine model of experimental pleurisy induced by the administration of carrageenan and formalin- fixed Staphylococcus aureus.
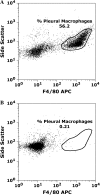
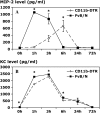
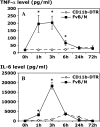
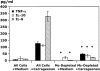
Measurements and main results: Conditional macrophage ablation mice express the human diphtheria toxin receptor under the control of the CD11b promoter such that the administration of diphtheria toxin induces ablation of nearly 97% of resident macrophages. Ablation of resident pleural macrophages before the administration of carrageenan or S. aureus dramatically reduced neutrophil influx into the pleural cavity. In the carrageenan model, the reduction in neutrophil infiltration was associated with marked early reduction in the level of macrophage inflammatory protein 2 as well as reduced levels of various cytokines, including tumor necrosis factor alpha, interleukin 6, and interleukin 10. Adoptive transfer of nontransgenic macrophages partially restored neutrophil infiltration. We also stimulated macrophage-depleted and nondepleted pleural cell populations with carrageenan in vitro and determined the production of chemokines and cytokines. Chemokine and cytokine production was markedly reduced by macrophage depletion, reinforcing the role of resident pleural macrophages in the generation of mediators that initiate acute inflammation.
Conclusion: These studies indicate a critical role for resident pleural macrophages in sensing perturbation to the local microenvironment and orchestrating subsequent neutrophil infiltration.
Figures






Similar articles
-
Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation.J Immunol. 2005 Feb 15;174(4):2336-42. doi: 10.4049/jimmunol.174.4.2336. J Immunol. 2005. PMID: 15699170
-
Nicorandil inhibits neutrophil recruitment in carrageenan-induced experimental pleurisy in mice.Eur J Pharmacol. 2015 Dec 15;769:306-12. doi: 10.1016/j.ejphar.2015.11.034. Epub 2015 Nov 25. Eur J Pharmacol. 2015. PMID: 26607465
-
Generation of inflammatory cytokines in zymosan-induced pleurisy in rats: TNF induces IL-6 and cytokine-induced neutrophil chemoattractant (CINC) in vivo.Cytokine. 1998 Dec;10(12):956-63. doi: 10.1006/cyto.1998.0376. Cytokine. 1998. PMID: 10049519
-
Immunobiology of pleural inflammation: potential implications for pathogenesis, diagnosis and therapy.Eur Respir J. 1997 Oct;10(10):2411-8. doi: 10.1183/09031936.97.10102411. Eur Respir J. 1997. PMID: 9387973 Review.
-
[Analysis of chemical mediators involved in acute inflammatory reaction with the rat pleurisy model].Nihon Yakurigaku Zasshi. 1997 Aug;110(2):59-68. doi: 10.1254/fpj.110.59. Nihon Yakurigaku Zasshi. 1997. PMID: 9306414 Review. Japanese.
Cited by
-
Interactions between the immune and nervous systems in pain.Nat Med. 2010 Nov;16(11):1267-76. doi: 10.1038/nm.2234. Epub 2010 Oct 14. Nat Med. 2010. PMID: 20948535 Free PMC article.
-
High Levels of MFG-E8 Confer a Good Prognosis in Prostate and Renal Cancer Patients.Cancers (Basel). 2022 Jun 4;14(11):2790. doi: 10.3390/cancers14112790. Cancers (Basel). 2022. PMID: 35681775 Free PMC article.
-
Nonclassical Ly6C(-) monocytes drive the development of inflammatory arthritis in mice.Cell Rep. 2014 Oct 23;9(2):591-604. doi: 10.1016/j.celrep.2014.09.032. Epub 2014 Oct 16. Cell Rep. 2014. PMID: 25373902 Free PMC article.
-
Immune targeting of the pleural space by intercostal approach.BMC Pulm Med. 2015 Feb 18;15:14. doi: 10.1186/s12890-015-0010-6. BMC Pulm Med. 2015. PMID: 25880308 Free PMC article.
-
Targeting macrophages in cancer immunotherapy.Signal Transduct Target Ther. 2021 Mar 26;6(1):127. doi: 10.1038/s41392-021-00506-6. Signal Transduct Target Ther. 2021. PMID: 33767177 Free PMC article.
References
-
- Kroegel C, Antony VB. Immunobiology of pleural inflammation: potential implications for pathogenesis, diagnosis and therapy. Eur Respir J 1997;10:2411–2418. - PubMed
-
- Antony VB. Immunological mechanisms in pleural disease. Eur Respir J 2003;21:539–544. - PubMed
-
- Antony VB, Hott JW, Kunkel SL, Godbey SW, Burdick MD, Strieter RM. Pleural mesothelial cell expression of C-C (monocyte chemotactic peptide) and C-X-C (interleukin 8) chemokines. Am J Respir Cell Mol Biol 1995;12:581–588. - PubMed
-
- Mohammed KA, Nasreen N, Ward MJ, Mubarak KK, Rodriguez-Panadero F, Antony VB. Mycobacterium-mediated chemokine expression in pleural mesothelial cells: role of C-C chemokines in tuberculous pleurisy. J Infect Dis 1998;178:1450–1456. - PubMed
-
- Mohammed KA, Nasreen N, Ward MJ, Antony VB. Macrophage inflammatory protein-1alpha C-C chemokine in parapneumonic pleural effusions. J Lab Clin Med 1998;132:202–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

