Ribosome exit tunnel can entropically stabilize alpha-helices
- PMID: 16357202
- PMCID: PMC1323178
- DOI: 10.1073/pnas.0508234102
Ribosome exit tunnel can entropically stabilize alpha-helices
Abstract
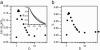
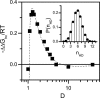
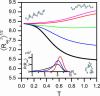
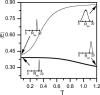
Several experiments have suggested that newly synthesized polypeptide chains can adopt helical structures deep within the ribosome exit tunnel. We hypothesize that confinement in the roughly cylindrical tunnel can entropically stabilize alpha-helices. The hypothesis is validated by using theory and simulations of coarse-grained off-lattice models. The model helix, which is unstable in the bulk, is stabilized in a cylindrical cavity provided the diameter (D) of the cylinder exceeds a critical value D*. When D < D* both the helical content and the helix-coil transition temperature (T(f)) decrease abruptly. Surprisingly, we find that the stability of the alpha-helix depends on the number (N) of amino acid residues. Entropic stabilization, as measured by changes in T(f), increases nonlinearly as N increases. The simulation results are in quantitative agreement with a standard helix-coil theory that takes into account entropy cost of confining a polypeptide chain in a cylinder. The results of this work are in qualitative accord with most of the findings of a recent experiment in which N-dependent ribosome-induced helix stabilization of transmembrane sequences was measured by fluorescence resonance energy transfer.
Figures

Similar articles
-
Protein translocation through a tunnel induces changes in folding kinetics: a lattice model study.Biotechnol Bioeng. 2006 May 5;94(1):105-17. doi: 10.1002/bit.20832. Biotechnol Bioeng. 2006. PMID: 16528757
-
Translocation of a beta-hairpin-forming peptide through a cylindrical tunnel.J Chem Phys. 2004 Nov 22;121(20):10268-77. doi: 10.1063/1.1807832. J Chem Phys. 2004. PMID: 15549903
-
Free energy determinants of secondary structure formation: I. alpha-Helices.J Mol Biol. 1995 Sep 22;252(3):351-65. doi: 10.1006/jmbi.1995.0502. J Mol Biol. 1995. PMID: 7563056
-
Helices and Sheets in vacuo.Phys Chem Chem Phys. 2007 Apr 14;9(14):1659-71. doi: 10.1039/b612615d. Epub 2007 Jan 19. Phys Chem Chem Phys. 2007. PMID: 17396176 Review.
-
Helix-coil transition theories. Are they correct?Acta Biochim Pol. 1997;44(3):423-32. Acta Biochim Pol. 1997. PMID: 9511955 Review.
Cited by
-
Atomic structure of anthrax protective antigen pore elucidates toxin translocation.Nature. 2015 May 28;521(7553):545-9. doi: 10.1038/nature14247. Epub 2015 Mar 16. Nature. 2015. PMID: 25778700 Free PMC article.
-
Charge, hydrophobicity, and confined water: putting past simulations into a simple theoretical framework.Biochem Cell Biol. 2010 Apr;88(2):359-69. doi: 10.1139/o09-187. Biochem Cell Biol. 2010. PMID: 20453936 Free PMC article.
-
Synthesis runs counter to directional folding of a nascent protein domain.Nat Commun. 2020 Oct 9;11(1):5096. doi: 10.1038/s41467-020-18921-8. Nat Commun. 2020. PMID: 33037221 Free PMC article.
-
Smoothing of the GB1 hairpin folding landscape by interfacial confinement.Biophys J. 2012 Aug 8;103(3):596-600. doi: 10.1016/j.bpj.2012.07.005. Biophys J. 2012. PMID: 22947876 Free PMC article.
-
Large-scale simulations of nucleoprotein complexes: ribosomes, nucleosomes, chromatin, chromosomes and CRISPR.Curr Opin Struct Biol. 2019 Apr;55:104-113. doi: 10.1016/j.sbi.2019.03.004. Epub 2019 May 21. Curr Opin Struct Biol. 2019. PMID: 31125796 Free PMC article. Review.
References
-
- Nissen, P., Hansen, J., Ban, N., Moore, P. B. & Steitz, T. A. (2000) Science 289, 920–930. - PubMed
-
- Harms, J., Schluenzen, F., Zarivach, R., Bashan, A., Gat, S., Agmon, I., Bartels, H., Franceschi, F. & Yonath, A. (2001) Cell 107, 679–688. - PubMed
-
- Milligan, R. A. & Unwin, P. N. (1986) Nature 319, 693–695. - PubMed
-
- Yonath, A., Leonard, K. R. & Wittman, H. G. (1987) Science 236, 813–816. - PubMed
-
- Gilbert, R. J. C., Fucini, P., Connell, S., Fuller, S. D., Nierhaus, K. H., Robinson, C. V., Dobson, C. M. & Stuart, D. I. (2004) Mol. Cell 14, 57–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

