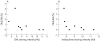Advanced glycation end product modification of bone proteins and bone remodelling: hypothesis and preliminary immunohistochemical findings
- PMID: 16344492
- PMCID: PMC1797982
- DOI: 10.1136/ard.2004.034348
Advanced glycation end product modification of bone proteins and bone remodelling: hypothesis and preliminary immunohistochemical findings
Abstract
Background: The process of bone remodelling is disturbed in the development of osteoporosis.
Objective: To investigate if proteins in osteoporotic bone are modified by advanced glycation end products (AGEs), and whether these alterations are related to measures of bone remodelling based on histomorphometric findings.
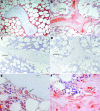
Methods: Bone specimens taken from the iliac crest by bone biopsy of eight osteoporotic patients were investigated by histomorphometry and by immunohistochemical staining with the AGEs imidazolone and N(epsilon)-carboxymethyllysine.
Results: Both AGEs were found in all bone specimens. The intensity of staining correlated with patient age. The percentage of bone surface covered with osteoblasts showed a significantly negative correlation with the staining intensity of both AGEs.
Conclusions: It is known that AGEs can regulate proliferation and differentiation of osteoblastic cells and that AGE-specific binding sites are present in cultured osteoblast-like cells. Moreover, AGE induced biological effects in these cells might be mediated by RAGE (receptor of AGE) or by other AGE receptors in different stages of osteoblast development. The inverse relation between AGE staining intensity and the percentage of bone surface covered with osteoblasts in the trabecular bone may provide evidence that AGE modification of bone proteins disturbs bone remodelling.
Similar articles
-
Advanced glycation end-products pentosidine and N epsilon-carboxymethyllysine are elevated in serum of patients with osteoporosis.Rheumatology (Oxford). 2003 Oct;42(10):1242-6. doi: 10.1093/rheumatology/keg324. Epub 2003 May 30. Rheumatology (Oxford). 2003. PMID: 12777635
-
Glycation endproducts in osteoporosis--is there a pathophysiologic importance?Clin Chim Acta. 2006 Sep;371(1-2):32-6. doi: 10.1016/j.cca.2006.03.017. Epub 2006 Jun 13. Clin Chim Acta. 2006. PMID: 16777084 Review.
-
Bone histomorphometry in the pathophysiological evaluation of primary and secondary osteoporosis and various treatment modalities.APMIS Suppl. 1995;51:1-44. APMIS Suppl. 1995. PMID: 7669370 Review.
-
[Bone histology in postmenopausal osteoporosis--variations in cellular activity].Acta Med Croatica. 2004;58(1):5-11. Acta Med Croatica. 2004. PMID: 15125387 Croatian.
-
Possible participation of advanced glycation end products in the pathogenesis of osteoporosis in diabetic patients.Med Hypotheses. 2005;65(6):1013-5. doi: 10.1016/j.mehy.2005.07.017. Med Hypotheses. 2005. PMID: 16146671
Cited by
-
Dietary Interventions for Type 2 Diabetes: How Millet Comes to Help.Front Plant Sci. 2016 Sep 27;7:1454. doi: 10.3389/fpls.2016.01454. eCollection 2016. Front Plant Sci. 2016. PMID: 27729921 Free PMC article. Review.
-
Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways.Bone. 2007 Feb;40(2):345-53. doi: 10.1016/j.bone.2006.09.011. Epub 2006 Oct 24. Bone. 2007. PMID: 17064973 Free PMC article.
-
Increased Advanced Glycation Endproducts, Stiffness, and Hardness in Iliac Crest Bone From Postmenopausal Women With Type 2 Diabetes Mellitus on Insulin.J Bone Miner Res. 2023 Feb;38(2):261-277. doi: 10.1002/jbmr.4757. Epub 2022 Dec 28. J Bone Miner Res. 2023. PMID: 36478472 Free PMC article.
-
Immunology of Osteoporosis: A Mini-Review.Gerontology. 2016;62(2):128-37. doi: 10.1159/000431091. Epub 2015 Jun 17. Gerontology. 2016. PMID: 26088283 Free PMC article. Review.
-
In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption.Bone. 2011 Aug;49(2):174-83. doi: 10.1016/j.bone.2011.04.009. Epub 2011 Apr 21. Bone. 2011. PMID: 21530698 Free PMC article.
References
-
- Parfitt A M. Quantum concept of bone remodeling and turnover: implications for the pathogenesis of osteoporosis. Calcif Tissue Int 1979281–5. - PubMed
-
- Teitelbaum S L, Ross P F. Genetic regulation of osteoclast development and function. Nat Rev Genet 20034638–649. - PubMed
-
- Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res 2001561–21. - PubMed
-
- Thornalley P J. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol (Noisy‐le‐grand) 1998441013–1023. - PubMed
-
- Vlassara H, Palace M R. Diabetes and advanced glycation endproducts. J Intern Med 200225187–101. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical