Golgi-mediated vacuolar sorting of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway
- PMID: 16339854
- PMCID: PMC1323493
- DOI: 10.1105/tpc.105.036665
Golgi-mediated vacuolar sorting of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway
Abstract
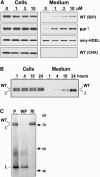
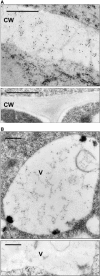
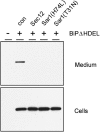
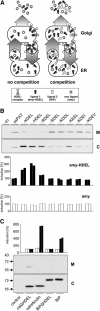
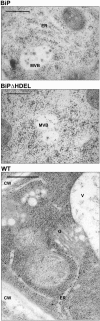
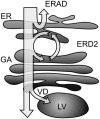
Quality control in the endoplasmic reticulum (ER) prevents the arrival of incorrectly or incompletely folded proteins at their final destinations and targets permanently misfolded proteins for degradation. Such proteins have a high affinity for the ER chaperone BiP and are finally degraded via retrograde translocation from the ER lumen back to the cytosol. This ER-associated protein degradation (ERAD) is currently thought to constitute the main disposal route, but there is growing evidence for a vacuolar role in quality control. We show that BiP is transported to the vacuole in a wortmannin-sensitive manner in tobacco (Nicotiana tabacum) and that it could play an active role in this second disposal route. ER export of BiP occurs via COPII-dependent transport to the Golgi apparatus, where it competes with other HDEL receptor ligands. When HDEL-mediated retrieval from the Golgi fails, BiP is transported to the lytic vacuole via multivesicular bodies, which represent the plant prevacuolar compartment. We also demonstrate that a subset of BiP-ligand complexes is destined to the vacuole and differs from those likely to be disposed of via the ERAD pathway. Vacuolar disposal could act in addition to ERAD to maximize the efficiency of quality control in the secretory pathway.
Figures


Similar articles
-
Protein quality control along the route to the plant vacuole.Plant Cell. 1997 Oct;9(10):1869-80. doi: 10.1105/tpc.9.10.1869. Plant Cell. 1997. PMID: 9368420 Free PMC article.
-
A vacuolar sorting domain may also influence the way in which proteins leave the endoplasmic reticulum.Plant Cell. 2001 Sep;13(9):2021-32. doi: 10.1105/tpc.000533. Plant Cell. 2001. PMID: 11549761 Free PMC article.
-
Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites.Plant Cell. 2005 May;17(5):1513-31. doi: 10.1105/tpc.104.026757. Epub 2005 Apr 1. Plant Cell. 2005. PMID: 15805489 Free PMC article.
-
The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends.J Biol Chem. 2019 Feb 8;294(6):2098-2108. doi: 10.1074/jbc.REV118.002804. Epub 2018 Dec 18. J Biol Chem. 2019. PMID: 30563838 Free PMC article. Review.
-
COPII and COPI traffic at the ER-Golgi interface.Physiology (Bethesda). 2011 Oct;26(5):348-64. doi: 10.1152/physiol.00017.2011. Physiology (Bethesda). 2011. PMID: 22013193 Review.
Cited by
-
Protective proteins are differentially expressed in tomato genotypes differing for their tolerance to low-temperature storage.Planta. 2010 Jul;232(2):483-500. doi: 10.1007/s00425-010-1184-z. Epub 2010 May 18. Planta. 2010. PMID: 20480178
-
Nanobody-based VSR7 tracing shows clathrin-dependent TGN to Golgi recycling.Nat Commun. 2023 Oct 30;14(1):6926. doi: 10.1038/s41467-023-42331-1. Nat Commun. 2023. PMID: 37903761 Free PMC article.
-
A recycling-defective vacuolar sorting receptor reveals an intermediate compartment situated between prevacuoles and vacuoles in tobacco.Plant Cell. 2010 Dec;22(12):3992-4008. doi: 10.1105/tpc.110.078436. Epub 2010 Dec 21. Plant Cell. 2010. PMID: 21177482 Free PMC article.
-
Coupled transport of Arabidopsis p24 proteins at the ER-Golgi interface.J Exp Bot. 2012 Jun;63(11):4243-61. doi: 10.1093/jxb/ers112. Epub 2012 May 10. J Exp Bot. 2012. PMID: 22577184 Free PMC article.
-
Targeting of the plant vacuolar sorting receptor BP80 is dependent on multiple sorting signals in the cytosolic tail.Plant Cell. 2006 Jun;18(6):1477-97. doi: 10.1105/tpc.105.040394. Epub 2006 May 19. Plant Cell. 2006. PMID: 16714388 Free PMC article.
References
-
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M.F., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77 895–907. - PubMed
-
- Ben-Zvi, A., De Los Rios, P., Dietler, G., and Goloubinoff, P. (2004). Active solubilization and refolding of stable protein aggregates by cooperative unfolding action of individual hsp70 chaperones. J. Biol. Chem. 279 37298–37303. - PubMed
-
- Blond-Elguindi, S., Cwirla, S.E., Dower, W.J., Lipshutz, R.J., Sprang, S.R., Sambrook, J.F., and Gething, M.J. (1993). Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell 75 717–728. - PubMed
-
- Brandizzi, F., Hanton, S., DaSilva, L.L., Boevink, P., Evans, D., Oparka, K., Denecke, J., and Hawes, C. (2003). ER quality control can lead to retrograde transport from the ER lumen to the cytosol and the nucleoplasm in plants. Plant J. 34 269–281. - PubMed
-
- Caldwell, S.R., Hill, K.J., and Cooper, A.A. (2001). Degradation of endoplasmic reticulum (ER) quality control substrates requires transport between the ER and Golgi. J. Biol. Chem. 276 23296–23303. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

