Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons
- PMID: 16339039
- PMCID: PMC6725905
- DOI: 10.1523/JNEUROSCI.3153-05.2005
Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons
Abstract
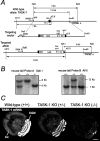
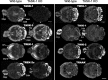
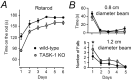
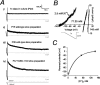
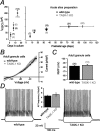
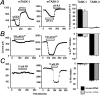
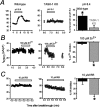
Two-pore domain potassium (K2P) channel expression is believed to underlie the developmental emergence of a potassium leak conductance [IK(SO)] in cerebellar granule neurons (CGNs), suggesting that K2P function is an important determinant of the input conductance and resting membrane potential. To investigate the role that different K2P channels may play in the regulation of CGN excitability, we generated a mouse lacking TASK-1, a K2P channel known to have high expression levels in CGNs. In situ hybridization and real-time PCR studies in wild-type and TASK-1 knock-outs (KOs) demonstrated that the expression of other K2P channels was unaltered in CGNs. TASK-1 knock-out mice were healthy and bred normally but exhibited compromised motor performance consistent with altered cerebellar function. Whole-cell recordings from adult cerebellar slice preparations revealed that the resting excitability of mature CGNs was no different in TASK-1 KO and littermate controls. However, the modulation of IK(SO) by extracellular Zn2+, ruthenium red, and H+ was altered. The IK(SO) recorded from TASK-1 knock-out CGNs was no longer sensitive to alkalization and was blocked by Zn2+ and ruthenium red. These results suggest that a TASK-1-containing channel population has been replaced by a homodimeric TASK-3 population in the TASK-1 knock-out. These data directly demonstrate that TASK-1 channels contribute to the properties of IK(SO) in adult CGNs. However, TASK channel subunit composition does not alter the resting excitability of CGNs but does influence sensitivity to endogenous modulators such as Zn2+ and H+.
Figures
Similar articles
-
A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons.Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3614-8. doi: 10.1073/pnas.97.7.3614. Proc Natl Acad Sci U S A. 2000. PMID: 10725353 Free PMC article.
-
Differential expression of two-pore domain potassium channels in rat cerebellar granule neurons.Biochem Biophys Res Commun. 2014 Oct 31;453(4):754-60. doi: 10.1016/j.bbrc.2014.10.012. Epub 2014 Oct 12. Biochem Biophys Res Commun. 2014. PMID: 25305496
-
Characterization of four types of background potassium channels in rat cerebellar granule neurons.J Physiol. 2002 Jul 15;542(Pt 2):431-44. doi: 10.1113/jphysiol.2002.017590. J Physiol. 2002. PMID: 12122143 Free PMC article.
-
What are the roles of the many different types of potassium channel expressed in cerebellar granule cells?Cerebellum. 2003;2(1):11-25. doi: 10.1080/14734220310015593. Cerebellum. 2003. PMID: 12882230 Review.
-
The TASK background K2P channels: chemo- and nutrient sensors.Trends Neurosci. 2007 Nov;30(11):573-80. doi: 10.1016/j.tins.2007.08.003. Epub 2007 Oct 22. Trends Neurosci. 2007. PMID: 17945357 Review.
Cited by
-
Potentiation of Gamma Aminobutyric Acid Receptors (GABAAR) by Ethanol: How Are Inhibitory Receptors Affected?Front Cell Neurosci. 2016 May 6;10:114. doi: 10.3389/fncel.2016.00114. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27199667 Free PMC article. Review.
-
Neuromodulation Enables Temperature Robustness and Coupling Between Fast and Slow Oscillator Circuits.Front Cell Neurosci. 2022 Mar 28;16:849160. doi: 10.3389/fncel.2022.849160. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35418838 Free PMC article.
-
Lysophosphatidic Acid and Several Neurotransmitters Converge on Rho-Kinase 2 Signaling to Manage Motoneuron Excitability.Front Mol Neurosci. 2021 Dec 6;14:788039. doi: 10.3389/fnmol.2021.788039. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34938160 Free PMC article.
-
Contractile and electrophysiological properties of pulmonary artery smooth muscle are not altered in TASK-1 knockout mice.J Physiol. 2011 Jul 1;589(Pt 13):3231-46. doi: 10.1113/jphysiol.2011.206748. Epub 2011 Apr 11. J Physiol. 2011. PMID: 21486782 Free PMC article.
-
Strain differences in pH-sensitive K+ channel-expressing cells in chemosensory and nonchemosensory brain stem nuclei.J Appl Physiol (1985). 2014 Oct 15;117(8):848-56. doi: 10.1152/japplphysiol.00439.2014. Epub 2014 Aug 21. J Appl Physiol (1985). 2014. PMID: 25150225 Free PMC article.
References
-
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S (1994) Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell 79: 377-388. - PubMed
-
- Aller MI, Jones A, Merlo D, Paterlini M, Meyer AH, Amtmann U, Brickley S, Jolin HE, McKenzie ANJ, Monyer H, Farrant M, Wisden W (2003) Cerebellar granule cell Cre recombinase expression. Genesis 36: 97-103. - PubMed
-
- Bayliss DA, Sirois JE, Talley EM (2003) The TASK family: two-pore domain background K+ channels. Mol Interv 3: 205-219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
