Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis
- PMID: 16330751
- PMCID: PMC1312421
- DOI: 10.1073/pnas.0509148102
Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis
Abstract
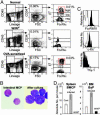
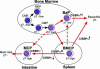
Basophils and mast cells, which are selectively endowed with the high-affinity IgE receptor and mediate a range of adaptive and innate immune responses, have an unknown developmental relationship. Here, by evaluating the expression of the beta7 integrin, a molecule that is required for selective homing of mast cell progenitors (MCPs) to the periphery, we identified bipotent progenitors that are capable of differentiating into either cell type in the mouse spleen. These basophil/mast cell progenitors (BMCPs) gave rise to basophils and mast cells at the single-cell level and reconstituted both mucosal and connective tissue mast cells. We also identified the basophil progenitor (BaP) and the MCP in the bone marrow and the gastrointestinal mucosa, respectively. We further show that the granulocyte-related transcription factor CCAAT/enhancer-binding protein alpha (C/EBPalpha) plays a primary role in the fate decision of BMCPs, being expressed in BaPs but not in MCPs. Thus, circulating basophils and tissue mast cells share a common developmental stage at which their fate decision might be controlled principally by C/EBPalpha.
Figures




Similar articles
-
The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages.Genes Dev. 2006 Nov 1;20(21):3010-21. doi: 10.1101/gad.1493506. Genes Dev. 2006. PMID: 17079688 Free PMC article.
-
IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen.J Immunol. 2009 Mar 1;182(5):2835-41. doi: 10.4049/jimmunol.0802870. J Immunol. 2009. PMID: 19234178 Free PMC article.
-
Antagonistic regulation by the transcription factors C/EBPα and MITF specifies basophil and mast cell fates.Immunity. 2013 Jul 25;39(1):97-110. doi: 10.1016/j.immuni.2013.06.012. Epub 2013 Jul 18. Immunity. 2013. PMID: 23871207 Free PMC article.
-
Regulation of basophil and mast cell development by transcription factors.Allergol Int. 2016 Apr;65(2):127-134. doi: 10.1016/j.alit.2016.01.006. Epub 2016 Mar 10. Allergol Int. 2016. PMID: 26972050 Review.
-
Mast cells: ontogeny, homing, and recruitment of a unique innate effector cell.J Allergy Clin Immunol. 2006 Jun;117(6):1285-91. doi: 10.1016/j.jaci.2006.04.017. J Allergy Clin Immunol. 2006. PMID: 16750988 Review.
Cited by
-
Basophils and Systemic Lupus Erythematosus in Murine Models and Human Patients.Biology (Basel). 2020 Sep 23;9(10):308. doi: 10.3390/biology9100308. Biology (Basel). 2020. PMID: 32977704 Free PMC article. Review.
-
Functional heterogeneity in the basophil cell lineage.Adv Immunol. 2012;115:141-59. doi: 10.1016/B978-0-12-394299-9.00005-9. Adv Immunol. 2012. PMID: 22608258 Free PMC article. Review.
-
IL-4-BATF signaling directly modulates IL-9 producing mucosal mast cell (MMC9) function in experimental food allergy.J Allergy Clin Immunol. 2021 Jan;147(1):280-295. doi: 10.1016/j.jaci.2020.08.043. Epub 2020 Oct 15. J Allergy Clin Immunol. 2021. PMID: 33069715 Free PMC article.
-
Potential effector and immunoregulatory functions of mast cells in mucosal immunity.Mucosal Immunol. 2015 May;8(3):444-63. doi: 10.1038/mi.2014.131. Epub 2015 Feb 11. Mucosal Immunol. 2015. PMID: 25669149 Free PMC article. Review.
-
Nutritional immunity: the impact of metals on lung immune cells and the airway microbiome during chronic respiratory disease.Respir Res. 2021 Apr 29;22(1):133. doi: 10.1186/s12931-021-01722-y. Respir Res. 2021. PMID: 33926483 Free PMC article. Review.
References
-
- Bochner, B. S. & Schleimer, R. P. (2001) Immunol. Rev. 179, 5-15. - PubMed
-
- Wedemeyer, J., Tsai, M. & Galli, S. J. (2000) Curr. Opin. Immunol. 12, 624-631. - PubMed
-
- Echtenacher, B., Mannel, D. N. & Hultner, L. (1996) Nature 381, 75-77. - PubMed
-
- Benoist, C. & Mathis, D. (2002) Nature 420, 875-878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

