Immunity by ubiquitylation: a reversible process of modification
- PMID: 16322747
- PMCID: PMC7096784
- DOI: 10.1038/nri1731
Immunity by ubiquitylation: a reversible process of modification
Abstract
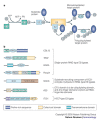
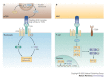
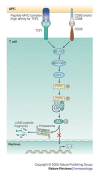
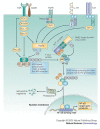
The conjugation of ubiquitin, a 76-amino-acid peptide, to a protein substrate provides a tag that either marks the labelled protein for degradation or modulates its function. The process of protein ubiquitylation--which is catalysed by coordinated enzymatic reactions that are mediated by enzymes known as E1, E2 and E3--has an important role in the modulation of immune responses. Importantly, protein ubiquitylation is a reversible process, and removal of ubiquitin molecules is mediated by de-ubiquitylating enzymes: for example, A20, which has been implicated in the regulation of immune responses. In addition, the conjugation of ubiquitin-like molecules, such as ISG15 (interferon-stimulated protein of 15 kDa), to proteins is also involved in immune regulation. This Review covers recent progress in our understanding of protein ubiquitylation in the immune system.
Conflict of interest statement
Josef Penninger holds shares in a company that plans to make CBL-B inhibitors.
Figures
Similar articles
-
The E3 ubiquitin ligase Itch in T cell activation, differentiation, and tolerance.Semin Immunol. 2007 Jun;19(3):197-205. doi: 10.1016/j.smim.2007.02.003. Epub 2007 Apr 11. Semin Immunol. 2007. PMID: 17433711 Free PMC article. Review.
-
Ubiquitin ligases and the immune response.Annu Rev Immunol. 2004;22:81-127. doi: 10.1146/annurev.immunol.22.012703.104813. Annu Rev Immunol. 2004. PMID: 15032575 Review.
-
Ubiquitylation and isgylation: overlapping enzymatic cascades do the job.Sci STKE. 2004 Aug 3;2004(245):pe43. doi: 10.1126/stke.2452004pe43. Sci STKE. 2004. PMID: 15304665 Review.
-
Deubiquitylation and regulation of the immune response.Nat Rev Immunol. 2008 Jul;8(7):501-11. doi: 10.1038/nri2337. Nat Rev Immunol. 2008. PMID: 18535581 Free PMC article. Review.
-
[Linear ubiquitination-mediated NF-κB regulation and its related diseases].Seikagaku. 2013 Jun;85(6):414-22. Seikagaku. 2013. PMID: 23875468 Review. Japanese. No abstract available.
Cited by
-
TNFAIP3 gene polymorphisms are associated with response to TNF blockade in psoriasis.J Invest Dermatol. 2012 Mar;132(3 Pt 1):593-600. doi: 10.1038/jid.2011.376. Epub 2011 Nov 24. J Invest Dermatol. 2012. PMID: 22113471 Free PMC article.
-
Global Analysis of O-GlcNAc Glycoproteins in Activated Human T Cells.J Immunol. 2016 Oct 15;197(8):3086-3098. doi: 10.4049/jimmunol.1502031. Epub 2016 Sep 21. J Immunol. 2016. PMID: 27655845 Free PMC article.
-
MCP-1-induced protein-1, an immune regulator.Protein Cell. 2012 Dec;3(12):903-10. doi: 10.1007/s13238-012-2075-9. Epub 2012 Nov 7. Protein Cell. 2012. PMID: 23132255 Free PMC article. Review.
-
Ubiquitination and proteasomal degradation of interferon regulatory factor-3 induced by Npro from a cytopathic bovine viral diarrhea virus.Virology. 2007 Sep 30;366(2):277-92. doi: 10.1016/j.virol.2007.04.023. Epub 2007 May 24. Virology. 2007. PMID: 17531282 Free PMC article.
-
Cbl-b in T-cell activation.Semin Immunopathol. 2010 Jun;32(2):137-48. doi: 10.1007/s00281-010-0197-9. Epub 2010 Feb 21. Semin Immunopathol. 2010. PMID: 20458601 Review.
References
-
- Pickart CM. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001;70:503–533. - PubMed
-
- Weissman AM. Themes and variations on ubiquitylation. Nature Rev. Mol. Cell Biol. 2001;2:169–178. - PubMed
-
- Liu YC. Ubiquitin ligases and the immune response. Annu. Rev. Immunol. 2004;22:81–127. - PubMed
-
- Hicke L. Protein regulation by monoubiquitin. Nature Rev. Mol. Cell Biol. 2001;2:195–201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

