Dimerization is required for SH3PX1 tyrosine phosphorylation in response to epidermal growth factor signalling and interaction with ACK2
- PMID: 16316319
- PMCID: PMC1383719
- DOI: 10.1042/BJ20050576
Dimerization is required for SH3PX1 tyrosine phosphorylation in response to epidermal growth factor signalling and interaction with ACK2
Abstract
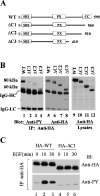
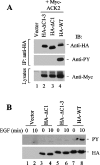
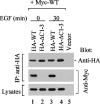
SH3PX1 [SNX9 (sorting nexin 9)] is a member of SNX super-family that is recognized by sharing a PX (phox homology) domain. We have previously shown that SH3PX1, phosphorylated by ACK2 (activated Cdc42-associated tyrosine kinase 2), regulates the degradation of EGF (epidermal growth factor) receptor. In mapping the tyrosine phosphorylation region, we found that the C-terminus of SH3PX1 is required for its tyrosine phosphorylation. Further analysis indicates that this region, known as the coiled-coil domain or the BAR (Bin-amphiphysin-Rvs homology) domain, is the dimerization domain of SH3PX1. Truncation of as little as 13 amino acid residues at the very C-terminus in the coiled-coil/BAR domain of SH3PX1 resulted in no dimerization, no ACK2-catalysed and EGF-stimulated tyrosine phosphorylation and no interaction with ACK2. The intracellular localization of SH3PX1 became dysfunctional upon truncation in the BAR domain. Taken together, our results indicate that the dimerization, which is mediated by the BAR domain, is essential for the intracellular function of SH3PX1.
Figures

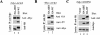

Similar articles
-
The Cdc42 target ACK2 interacts with sorting nexin 9 (SH3PX1) to regulate epidermal growth factor receptor degradation.J Biol Chem. 2002 Mar 22;277(12):10134-8. doi: 10.1074/jbc.M110329200. Epub 2002 Jan 17. J Biol Chem. 2002. PMID: 11799118
-
Interaction of activated Cdc42-associated tyrosine kinase ACK2 with HSP90.Biochem J. 2004 Aug 15;382(Pt 1):199-204. doi: 10.1042/BJ20040623. Biochem J. 2004. PMID: 15144235 Free PMC article.
-
Sorting nexin 16 regulates EGF receptor trafficking by phosphatidylinositol-3-phosphate interaction with the Phox domain.J Cell Sci. 2004 Aug 15;117(Pt 18):4209-18. doi: 10.1242/jcs.01233. Epub 2004 Aug 3. J Cell Sci. 2004. PMID: 15292396
-
SNX9 as an adaptor for linking synaptojanin-1 to the Cdc42 effector ACK1.FEBS Lett. 2005 Sep 12;579(22):5040-8. doi: 10.1016/j.febslet.2005.07.093. FEBS Lett. 2005. PMID: 16137687
-
Autoregulatory mechanisms in protein-tyrosine kinases.J Biol Chem. 1998 May 15;273(20):11987-90. doi: 10.1074/jbc.273.20.11987. J Biol Chem. 1998. PMID: 9575136 Review. No abstract available.
Cited by
-
The unique mechanism of SNX9 BAR domain for inducing membrane tubulation.Mol Cells. 2014 Oct 31;37(10):753-8. doi: 10.14348/molcells.2014.0228. Epub 2014 Sep 26. Mol Cells. 2014. PMID: 25256216 Free PMC article.
-
Rapid mapping of interactions between Human SNX-BAR proteins measured in vitro by AlphaScreen and single-molecule spectroscopy.Mol Cell Proteomics. 2014 Sep;13(9):2233-45. doi: 10.1074/mcp.M113.037275. Epub 2014 May 27. Mol Cell Proteomics. 2014. PMID: 24866125 Free PMC article.
-
The PX-BAR membrane-remodeling unit of sorting nexin 9.EMBO J. 2007 Nov 14;26(22):4788-800. doi: 10.1038/sj.emboj.7601889. Epub 2007 Oct 18. EMBO J. 2007. PMID: 17948057 Free PMC article.
-
Interaction of the Wiskott-Aldrich syndrome protein with sorting nexin 9 is required for CD28 endocytosis and cosignaling in T cells.Proc Natl Acad Sci U S A. 2007 Jan 30;104(5):1593-8. doi: 10.1073/pnas.0610543104. Epub 2007 Jan 22. Proc Natl Acad Sci U S A. 2007. PMID: 17242350 Free PMC article.
-
Sorting nexin 9 negatively regulates invadopodia formation and function in cancer cells.J Cell Sci. 2016 Jul 15;129(14):2804-16. doi: 10.1242/jcs.188045. Epub 2016 Jun 8. J Cell Sci. 2016. PMID: 27278018 Free PMC article.
References
-
- Bravo J., Karathanassis D., Pacold C. M., Pacold M. E., Ellson C. D., Anderson K. E., Butler P. J., Lavenir I., Perisic O., Hawkins P. T., et al. The crystal structure of the PX domain from p40phox bound to phosphatidylinositol 3-phosphate. Mol. Cell. 2001;8:829–839. - PubMed
-
- Kanai F., Liu H., Field S. J., Akbary H., Matsuo T., Brown G. E., Cantley L. C., Yaffe M. B. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat. Cell Biol. 2001;3:675–678. - PubMed
-
- Xu Y., Hortsman H., Seet L., Wong S. H., Hong W. SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat. Cell Biol. 2001;3:658–666. - PubMed
-
- Kurten R. C., Cadena D. L., Gill G. N. Enhanced degradation of EGFRs by a sorting nexin, SNX1. Science. 1996;272:1008–1010. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

