Preferential migration of effector CD8+ T cells into the interstitium of the normal lung
- PMID: 16308575
- PMCID: PMC1288831
- DOI: 10.1172/JCI24482
Preferential migration of effector CD8+ T cells into the interstitium of the normal lung
Abstract
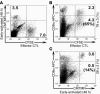
The respiratory tract is a primary site of infection and exposure to environmental antigens and an important site of memory T cell localization. We analyzed the migration and retention of naive and activated CD8+ T cells within the noninflamed lungs and quantitated the partitioning of adoptively transferred T cells between the pulmonary vascular and interstitial compartments. Activated but not naive T cells were retained within the lungs for a prolonged period. Effector CD8+ T cells preferentially egressed from the pulmonary vascular compartment into the noninflamed pulmonary interstitium. T cell retention within the lung vasculature was leukocyte function antigen-1 dependent, while the egress of effector T cells from the vascular to the interstitium functions through a pertussis toxin-sensitive (PTX-sensitive) mechanism driven in part by constitutive CC chemokine ligand 5 expression in the lungs. These results document a novel mechanism of adhesion receptor- and pulmonary chemokine-dependent regulation of the migration of activated CD8+ T cells into an important nonlymphoid peripheral site (i.e., the normal/noninflamed lung).
Figures




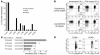
Similar articles
-
Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin.J Immunol. 2004 Apr 15;172(8):4875-82. doi: 10.4049/jimmunol.172.8.4875. J Immunol. 2004. PMID: 15067066
-
CD4+CD25+Foxp3+ regulatory T cells are dispensable for controlling CD8+ T cell-mediated lung inflammation.J Immunol. 2011 Jun 1;186(11):6106-18. doi: 10.4049/jimmunol.1000632. Epub 2011 Apr 25. J Immunol. 2011. PMID: 21518973
-
LFA-1 is required for retention of effector CD8 T cells in mouse lungs.Blood. 2003 Jun 15;101(12):4916-22. doi: 10.1182/blood-2002-10-3159. Epub 2003 Mar 6. Blood. 2003. PMID: 12623847
-
Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues.Mol Immunol. 2005 May;42(7):799-809. doi: 10.1016/j.molimm.2004.06.040. Epub 2004 Nov 23. Mol Immunol. 2005. PMID: 15829268 Review.
-
T-lymphocytes and cytokines in sarcoidosis.Curr Opin Pulm Med. 2002 Sep;8(5):435-40. doi: 10.1097/00063198-200209000-00016. Curr Opin Pulm Med. 2002. PMID: 12172449 Review.
Cited by
-
Tissue-resident memory T cells.Immunity. 2014 Dec 18;41(6):886-97. doi: 10.1016/j.immuni.2014.12.007. Epub 2014 Dec 6. Immunity. 2014. PMID: 25526304 Free PMC article. Review.
-
Cervicovaginal Tissue Residence Confers a Distinct Differentiation Program upon Memory CD8 T Cells.J Immunol. 2021 Jun 15;206(12):2937-2948. doi: 10.4049/jimmunol.2100166. Epub 2021 Jun 4. J Immunol. 2021. PMID: 34088770 Free PMC article.
-
The migration of T cells in response to influenza virus is altered in neonatal mice.J Immunol. 2010 Sep 1;185(5):2980-8. doi: 10.4049/jimmunol.0903075. Epub 2010 Jul 23. J Immunol. 2010. PMID: 20656925 Free PMC article.
-
The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections.Immunity. 2008 Jul 18;29(1):101-13. doi: 10.1016/j.immuni.2008.05.011. Immunity. 2008. PMID: 18617426 Free PMC article.
-
Tissue-Resident T Cells and Other Resident Leukocytes.Annu Rev Immunol. 2019 Apr 26;37:521-546. doi: 10.1146/annurev-immunol-042617-053214. Epub 2019 Feb 6. Annu Rev Immunol. 2019. PMID: 30726153 Free PMC article. Review.
References
-
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. - PubMed
-
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. - PubMed
-
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. - PubMed
-
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

