The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA
- PMID: 16306406
- PMCID: PMC6725884
- DOI: 10.1523/JNEUROSCI.2029-05.2005
The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA
Abstract
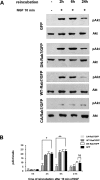
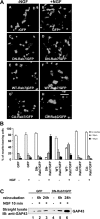
Nerve growth factor (NGF) and its TrkA receptor exert important bioactivities on neuronal cells such as promoting survival and neurite outgrowth. Activated TrkA receptors are not only localized on the cell surface but also in signaling endosomes, and internalized TrkA receptors are important for the mediation of neurite outgrowth. The regulation of the endosomal trafficking of TrkA is so far unknown. Because the endosome-associated GTPase Rab7 coimmunoprecipitated with TrkA, we examined whether the endosomal trafficking of TrkA might be under the control of Rab7. Inhibiting Rab7 by expression of a green fluorescent protein-tagged, dominant-negative Rab7 variant resulted in endosomal accumulation of TrkA and pronounced enhancement of TrkA signaling in response to limited stimulations with NGF, such as increased activation of Erk1/2 (extracellular signal-regulated kinase 1/2), neurite outgrowth, and expression of GAP-43 (growth-associated protein 43). Our studies show that the endosomal GTPase Rab7 controls the endosomal trafficking and neurite outgrowth signaling of TrkA. Because mutations of Rab7 are found in patients suffering from hereditary polyneuropathies, dysfunction of Rab7 might contribute to neurodegenerative conditions by affecting the trafficking of neurotrophins. Moreover, strategies aimed at controlling Rab7 activity might be useful for the treatment of neurodegenerative diseases.
Figures


Similar articles
-
Endophilin B1 as a novel regulator of nerve growth factor/ TrkA trafficking and neurite outgrowth.J Neurosci. 2008 Sep 3;28(36):9002-12. doi: 10.1523/JNEUROSCI.0767-08.2008. J Neurosci. 2008. PMID: 18768694 Free PMC article.
-
Endosomal acidification by Na+/H+ exchanger NHE5 regulates TrkA cell-surface targeting and NGF-induced PI3K signaling.Mol Biol Cell. 2013 Nov;24(21):3435-48. doi: 10.1091/mbc.E12-06-0445. Epub 2013 Sep 4. Mol Biol Cell. 2013. PMID: 24006492 Free PMC article.
-
Rab22 controls NGF signaling and neurite outgrowth in PC12 cells.Mol Biol Cell. 2011 Oct;22(20):3853-60. doi: 10.1091/mbc.E11-03-0277. Epub 2011 Aug 17. Mol Biol Cell. 2011. PMID: 21849477 Free PMC article.
-
Regulation of Endosomal Trafficking by Rab7 and Its Effectors in Neurons: Clues from Charcot-Marie-Tooth 2B Disease.Biomolecules. 2023 Sep 16;13(9):1399. doi: 10.3390/biom13091399. Biomolecules. 2023. PMID: 37759799 Free PMC article. Review.
-
Biogenesis and function of the NGF/TrkA signaling endosome.Int Rev Cell Mol Biol. 2015;314:239-57. doi: 10.1016/bs.ircmb.2014.10.002. Epub 2014 Nov 18. Int Rev Cell Mol Biol. 2015. PMID: 25619719 Free PMC article. Review.
Cited by
-
Endosome-mediated retrograde axonal transport of P2X3 receptor signals in primary sensory neurons.Cell Res. 2012 Apr;22(4):677-96. doi: 10.1038/cr.2011.197. Epub 2011 Dec 13. Cell Res. 2012. PMID: 22157653 Free PMC article.
-
Alteration of the late endocytic pathway in Charcot-Marie-Tooth type 2B disease.Cell Mol Life Sci. 2021 Jan;78(1):351-372. doi: 10.1007/s00018-020-03510-1. Epub 2020 Apr 13. Cell Mol Life Sci. 2021. PMID: 32280996 Free PMC article.
-
Retrograde nerve growth factor signaling abnormalities in familial dysautonomia.J Clin Invest. 2020 May 1;130(5):2478-2487. doi: 10.1172/JCI130401. J Clin Invest. 2020. PMID: 32281946 Free PMC article.
-
Alterations of autophagy in the peripheral neuropathy Charcot-Marie-Tooth type 2B.Autophagy. 2018;14(6):930-941. doi: 10.1080/15548627.2017.1388475. Epub 2018 May 4. Autophagy. 2018. PMID: 29130394 Free PMC article.
-
Visualizing directional Rab7 and TrkA cotrafficking in axons by pTIRF microscopy.Methods Mol Biol. 2015;1298:319-29. doi: 10.1007/978-1-4939-2569-8_27. Methods Mol Biol. 2015. PMID: 25800854 Free PMC article.
References
-
- Barbieri MA, Fernandez-Pol S, Hunker C, Horazdovsky BH, Stahl PD (2004) Role of rab5 in EGF receptor-mediated signal transduction. Eur J Cell Biol 83: 305-314. - PubMed
-
- Bernd P, Greene LA (1984) Association of 125I-nerve growth factor with PC12 pheochromocytoma cells. Evidence for internalization via high-affinity receptors only and for long-term regulation by nerve growth factor of both high- and low-affinity receptors. J Biol Chem 259: 15509-15516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
