M-CSF mediates TNF-induced inflammatory osteolysis
- PMID: 16294221
- PMCID: PMC1283943
- DOI: 10.1172/JCI26132
M-CSF mediates TNF-induced inflammatory osteolysis
Abstract
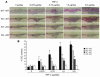
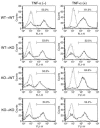
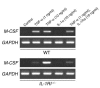
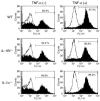
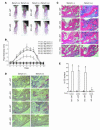
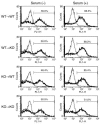
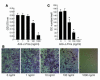
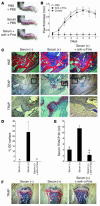
TNF-alpha is the dominant cytokine in inflammatory osteolysis. Using mice whose BM stromal cells and osteoclast precursors are chimeric for the presence of TNF receptors, we found that both cell types mediated the cytokine's osteoclastogenic properties. The greater contribution was made, however, by stromal cells that express the osteoclastogenic cytokine M-CSF. TNF-alpha stimulated M-CSF gene expression, in vivo, only in the presence of TNF-responsive stromal cells. M-CSF, in turn, induced the key osteoclastogenic cytokine receptor, receptor activator of NF-kappaB (RANK), in osteoclast precursors. In keeping with the proproliferative and survival properties of M-CSF, TNF-alpha enhanced osteoclast precursor number only in the presence of stromal cells bearing TNF receptors. To determine the clinical relevance of these observations, we induced inflammatory arthritis in wild-type mice and treated them with a mAb directed against the M-CSF receptor, c-Fms. Anti-c-Fms mAb selectively and completely arrested the profound pathological osteoclastogenesis attending this condition, the significance of which is reflected by similar blunting of the in vivo bone resorption marker tartrate-resistant acid phosphatase 5b (TRACP 5b). Confirming that inhibition of the M-CSF signaling pathway targets TNF-alpha, anti-c-Fms also completely arrested osteolysis in TNF-injected mice with nominal effect on macrophage number. M-CSF and its receptor, c-Fms, therefore present as candidate therapeutic targets in states of inflammatory bone erosion.
Figures

Similar articles
-
Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse.J Bone Miner Res. 2005 Sep;20(9):1659-68. doi: 10.1359/JBMR.050503. Epub 2005 May 2. J Bone Miner Res. 2005. PMID: 16059637
-
Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction.J Exp Med. 2000 Jan 17;191(2):275-86. doi: 10.1084/jem.191.2.275. J Exp Med. 2000. PMID: 10637272 Free PMC article.
-
Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor.J Clin Invest. 1997 Sep 15;100(6):1557-65. doi: 10.1172/JCI119679. J Clin Invest. 1997. PMID: 9294124 Free PMC article.
-
Canonical and non-canonical pathways of osteoclast formation.Histol Histopathol. 2009 Mar;24(3):337-46. doi: 10.14670/HH-24.337. Histol Histopathol. 2009. PMID: 19130404 Review.
-
Tartrate-resistant acid phosphatase knockout mice.J Bone Miner Res. 2003 Oct;18(10):1905-7. doi: 10.1359/jbmr.2003.18.10.1905. J Bone Miner Res. 2003. PMID: 14584904 Review.
Cited by
-
Immunological reaction in TNF-α-mediated osteoclast formation and bone resorption in vitro and in vivo.Clin Dev Immunol. 2013;2013:181849. doi: 10.1155/2013/181849. Epub 2013 May 23. Clin Dev Immunol. 2013. PMID: 23762085 Free PMC article. Review.
-
Immunomodulatory drug methotrexate used to treat patients with chronic inflammatory rheumatisms post-chikungunya does not impair the synovial antiviral and bone repair responses.PLoS Negl Trop Dis. 2018 Aug 3;12(8):e0006634. doi: 10.1371/journal.pntd.0006634. eCollection 2018 Aug. PLoS Negl Trop Dis. 2018. PMID: 30074983 Free PMC article.
-
Tussilagone Inhibits Osteoclastogenesis and Periprosthetic Osteolysis by Suppressing the NF-κB and P38 MAPK Signaling Pathways.Front Pharmacol. 2020 Apr 3;11:385. doi: 10.3389/fphar.2020.00385. eCollection 2020. Front Pharmacol. 2020. PMID: 32317967 Free PMC article.
-
Updates on Osteoimmunology: What's New on the Cross-Talk Between Bone and Immune System.Front Endocrinol (Lausanne). 2019 Apr 18;10:236. doi: 10.3389/fendo.2019.00236. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31057482 Free PMC article. Review.
-
IRAK4 inhibition: a promising strategy for treating RA joint inflammation and bone erosion.Cell Mol Immunol. 2021 Sep;18(9):2199-2210. doi: 10.1038/s41423-020-0433-8. Epub 2020 May 15. Cell Mol Immunol. 2021. PMID: 32415262 Free PMC article.
References
-
- Lacey DL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. - PubMed
-
- Bekker PJ, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J. Bone Miner. Res. 2004;19:1059–1066. - PubMed
-
- Feldmann M, Maini RN. Anti-TNF therapy of rheumatoid arthritis: what have we learned? Ann. Rev. Immunol. 2001;19:163–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK056341/DK/NIDDK NIH HHS/United States
- AR032788/AR/NIAMS NIH HHS/United States
- R01 AR046523/AR/NIAMS NIH HHS/United States
- R37 AR046523/AR/NIAMS NIH HHS/United States
- AR046852/AR/NIAMS NIH HHS/United States
- AR046523/AR/NIAMS NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R01 AR046852/AR/NIAMS NIH HHS/United States
- AR048853/AR/NIAMS NIH HHS/United States
- R01 AR032788/AR/NIAMS NIH HHS/United States
- R01 AR048812/AR/NIAMS NIH HHS/United States
- R01 AR048853/AR/NIAMS NIH HHS/United States
- AR048812/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

