Phospholipase D2 is required for efficient endocytic recycling of transferrin receptors
- PMID: 16291863
- PMCID: PMC1356572
- DOI: 10.1091/mbc.e05-05-0389
Phospholipase D2 is required for efficient endocytic recycling of transferrin receptors
Abstract
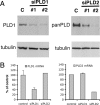
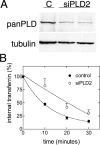
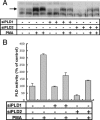
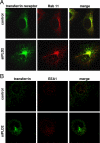
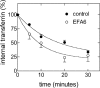
RNA interference-mediated depletion of phospholipase D2 (PLD2), but not PLD1, inhibited recycling of transferrin receptors in HeLa cells, whereas the internalization rate was unaffected by depletion of either PLD. Although reduction of both PLD isoforms inhibits PLD activity stimulated by phorbol 12-myristic 13-acetate, only depletion of PLD2 decreased nonstimulated activity. Cells with reduced PLD2 accumulated a greater fraction of transferrin receptors in a perinuclear compartment that was positive for Rab11, a marker of recycling endosomes. EFA6, an exchange factor for Arf6, has been proposed to stimulate the recycling of transferrin receptors. Thus, one consequence of EFA6 overexpression would be a reduction of the internal pool of receptors. We confirmed this observation in control HeLa cells; however, overexpression of EFA6 failed to decrease the internal pool of transferrin receptors that accumulate in cells previously depleted of PLD2. These observations suggest that either PLD2 is required for a constitutive Arf6-mediated recycling pathway or in the absence of PLD2 transferrin receptors accumulate in recycling endosomes that are not responsive to overexpression of EFA6.
Figures


Similar articles
-
Localization and regulation of phospholipase D2 by ARF6.J Cell Biochem. 2005 May 1;95(1):149-64. doi: 10.1002/jcb.20351. J Cell Biochem. 2005. PMID: 15759270
-
Studies of the roles of ADP-ribosylation factors and phospholipase D in phorbol ester-induced membrane ruffling.J Cell Physiol. 2005 Feb;202(2):608-22. doi: 10.1002/jcp.20156. J Cell Physiol. 2005. PMID: 15389577
-
ARF1 and ARF3 are required for the integrity of recycling endosomes and the recycling pathway.Cell Struct Funct. 2012;37(2):141-54. doi: 10.1247/csf.12015. Epub 2012 Sep 5. Cell Struct Funct. 2012. PMID: 22971977
-
Signalling role for ARF and phospholipase D in mast cell exocytosis stimulated by crosslinking of the high affinity FcepsilonR1 receptor.Mol Immunol. 2002 Sep;38(16-18):1277-82. doi: 10.1016/s0161-5890(02)00075-5. Mol Immunol. 2002. PMID: 12217395 Review.
-
The regulation of phospholipase D by inositol phospholipids and small GTPases.FEBS Lett. 2002 Oct 30;531(1):62-4. doi: 10.1016/s0014-5793(02)03410-5. FEBS Lett. 2002. PMID: 12401204 Review.
Cited by
-
Walnut extract inhibits LPS-induced activation of BV-2 microglia via internalization of TLR4: possible involvement of phospholipase D2.Inflammation. 2010 Oct;33(5):325-33. doi: 10.1007/s10753-010-9189-0. Inflammation. 2010. PMID: 20213499
-
Mechanism of membrane fusion: protein-protein interaction and beyond.Int J Physiol Pathophysiol Pharmacol. 2019 Dec 15;11(6):250-257. eCollection 2019. Int J Physiol Pathophysiol Pharmacol. 2019. PMID: 31993099 Free PMC article. Review.
-
Role of lipids and actin in the formation of clathrin-coated pits.Exp Cell Res. 2006 Dec 10;312(20):4036-48. doi: 10.1016/j.yexcr.2006.09.025. Epub 2006 Sep 30. Exp Cell Res. 2006. PMID: 17097636 Free PMC article.
-
Phospholipase D signaling: orchestration by PIP2 and small GTPases.Naunyn Schmiedebergs Arch Pharmacol. 2007 Feb;374(5-6):399-411. doi: 10.1007/s00210-007-0131-4. Epub 2007 Jan 24. Naunyn Schmiedebergs Arch Pharmacol. 2007. PMID: 17245604 Free PMC article. Review.
-
Phospholipase D is involved in the formation of Golgi associated clathrin coated vesicles in human parotid duct cells.PLoS One. 2014 Mar 11;9(3):e91868. doi: 10.1371/journal.pone.0091868. eCollection 2014. PLoS One. 2014. PMID: 24618697 Free PMC article.
References
-
- Barbieri, M. A., Heath, C. M., Peters, E. M., Wells, A., Davis, J. N., and Stahl, P. D. (2001). Phosphatidylinositol-4-phosphate 5-kinase-1beta is essential for epidermal growth factor receptor-mediated endocytosis. J. Biol. Chem. 276, 47212-47216. - PubMed
-
- Bi, K., Roth, M. G., and Ktistakis, N. T. (1997). Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr. Biol. 7, 301-307. - PubMed
-
- Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911-917. - PubMed
-
- Brown, H. A., Gutowski, S., Moomaw, C. R., Slaughter, C., and Sternweis, P. C. (1993). ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell 75, 1137-1144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

