Induction of neutralizing antibodies against human immunodeficiency virus type 1 primary isolates by Gag-Env pseudovirion immunization
- PMID: 16282480
- PMCID: PMC1287556
- DOI: 10.1128/JVI.79.23.14804-14814.2005
Induction of neutralizing antibodies against human immunodeficiency virus type 1 primary isolates by Gag-Env pseudovirion immunization
Abstract
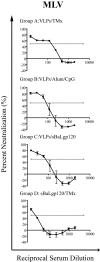
A major challenge for the development of an effective HIV vaccine is to elicit neutralizing antibodies against a broad array of primary isolates. Monomeric gp120-based vaccine approaches have not been successful in inducing this type of response, prompting a number of approaches designed to recreate the native glycoprotein complex that exists on the viral membrane. Gag-Env pseudovirions are noninfectious viruslike particles that recreate the native envelope glycoprotein structure and have the potential to generate neutralizing antibody responses against primary isolates. In this study, an inducible cell line was created in order to generate Gag-Env pseudovirions for examination of neutralizing antibody responses in guinea pigs. Unadjuvanted pseudovirions generated relatively weak anti-gp120 responses, while the use of a block copolymer water-in-oil emulsion or aluminum hydroxide combined with CpG oligodeoxynucleotides resulted in high levels of antibodies that bind to gp120. Sera from immunized animals neutralized a panel of human immunodeficiency virus (HIV) type 1 primary isolate viruses at titers that were significantly higher than that of the corresponding monomeric gp120 protein. Interpretation of these results was complicated by the occurrence of neutralizing antibodies directed against cellular (non-envelope protein) components of the pseudovirion. However, a major component of the pseudovirion-elicited antibody response was directed specifically against the HIV envelope. These results provide support for the role of pseudovirion-based vaccines in generating neutralizing antibodies against primary isolates of HIV and highlight the potential confounding role of antibodies directed at non-envelope cell surface components.
Figures



Similar articles
-
Comparison of HIV Type 1 ADA gp120 monomers versus gp140 trimers as immunogens for the induction of neutralizing antibodies.AIDS Res Hum Retroviruses. 2005 Jan;21(1):58-67. doi: 10.1089/aid.2005.21.58. AIDS Res Hum Retroviruses. 2005. PMID: 15665645
-
Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants.J Virol. 2006 Feb;80(3):1414-26. doi: 10.1128/JVI.80.3.1414-1426.2006. J Virol. 2006. PMID: 16415019 Free PMC article.
-
Neutralizing antibodies from the sera of human immunodeficiency virus type 1-infected individuals bind to monomeric gp120 and oligomeric gp140.J Virol. 1998 Dec;72(12):9656-67. doi: 10.1128/JVI.72.12.9656-9667.1998. J Virol. 1998. PMID: 9811699 Free PMC article.
-
Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design.Immunol Lett. 1997 Jun 1;57(1-3):105-12. doi: 10.1016/s0165-2478(97)00043-6. Immunol Lett. 1997. Corrected and republished in: Immunol Lett. 1997 Jul;58(2):125-32. doi: 10.1016/s0165-2478(97)00109-0. PMID: 9232434 Corrected and republished. Review.
-
Erratum to "Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design".Immunol Lett. 1997 Jul;58(2):125-32. doi: 10.1016/s0165-2478(97)00109-0. Immunol Lett. 1997. PMID: 9271324 Review.
Cited by
-
Neutralizing antibodies to HIV-1 induced by immunization.J Exp Med. 2013 Feb 11;210(2):209-23. doi: 10.1084/jem.20121827. J Exp Med. 2013. PMID: 23401570 Free PMC article. Review.
-
Immunogenicity of HIV-1-Based Virus-Like Particles with Increased Incorporation and Stability of Membrane-Bound Env.Vaccines (Basel). 2021 Mar 10;9(3):239. doi: 10.3390/vaccines9030239. Vaccines (Basel). 2021. PMID: 33801906 Free PMC article.
-
Repeated Vaccination of Cows with HIV Env gp140 during Subsequent Pregnancies Elicits and Sustains an Enduring Strong Env-Binding and Neutralising Antibody Response.PLoS One. 2016 Jun 14;11(6):e0157353. doi: 10.1371/journal.pone.0157353. eCollection 2016. PLoS One. 2016. PMID: 27300145 Free PMC article.
-
Multivalent benzoboroxole functionalized polymers as gp120 glycan targeted microbicide entry inhibitors.Mol Pharm. 2010 Feb 1;7(1):116-29. doi: 10.1021/mp900159n. Mol Pharm. 2010. PMID: 20014858 Free PMC article.
-
Enzyme digests eliminate nonfunctional Env from HIV-1 particle surfaces, leaving native Env trimers intact and viral infectivity unaffected.J Virol. 2011 Jun;85(12):5825-39. doi: 10.1128/JVI.00154-11. Epub 2011 Apr 6. J Virol. 2011. PMID: 21471242 Free PMC article.
References
-
- Anonymous. 2004. UNAIDS/WHO global AIDS statistics. AIDS Care 16:1050.
-
- Arthur, L. O., J. W. Bess, Jr., R. C. Sowder II, R. E. Benveniste, D. L. Mann, J. C. Chermann, and L. E. Henderson. 1992. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science 258:1935-1938. - PubMed
-
- Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. - PubMed
-
- Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:2019-2035. - PubMed
-
- Burton, D. R., and D. C. Montefiori. 1997. The antibody response in HIV-1 infection. AIDS 11(Suppl. A):S87-S98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P60 DK020593/DK/NIDDK NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 AI052007/AI/NIAID NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- R01 AI52007/AI/NIAID NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

