Alkaline pH homeostasis in bacteria: new insights
- PMID: 16277975
- PMCID: PMC3072713
- DOI: 10.1016/j.bbamem.2005.09.010
Alkaline pH homeostasis in bacteria: new insights
Abstract
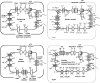
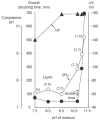
The capacity of bacteria to survive and grow at alkaline pH values is of widespread importance in the epidemiology of pathogenic bacteria, in remediation and industrial settings, as well as in marine, plant-associated and extremely alkaline ecological niches. Alkali-tolerance and alkaliphily, in turn, strongly depend upon mechanisms for alkaline pH homeostasis, as shown in pH shift experiments and growth experiments in chemostats at different external pH values. Transcriptome and proteome analyses have recently complemented physiological and genetic studies, revealing numerous adaptations that contribute to alkaline pH homeostasis. These include elevated levels of transporters and enzymes that promote proton capture and retention (e.g., the ATP synthase and monovalent cation/proton antiporters), metabolic changes that lead to increased acid production, and changes in the cell surface layers that contribute to cytoplasmic proton retention. Targeted studies over the past decade have followed up the long-recognized importance of monovalent cations in active pH homeostasis. These studies show the centrality of monovalent cation/proton antiporters in this process while microbial genomics provides information about the constellation of such antiporters in individual strains. A comprehensive phylogenetic analysis of both eukaryotic and prokaryotic genome databases has identified orthologs from bacteria to humans that allow better understanding of the specific functions and physiological roles of the antiporters. Detailed information about the properties of multiple antiporters in individual strains is starting to explain how specific monovalent cation/proton antiporters play dominant roles in alkaline pH homeostasis in cells that have several additional antiporters catalyzing ostensibly similar reactions. New insights into the pH-dependent Na(+)/H(+) antiporter NhaA that plays an important role in Escherichia coli have recently emerged from the determination of the structure of NhaA. This review highlights the approaches, major findings and unresolved problems in alkaline pH homeostasis, focusing on the small number of well-characterized alkali-tolerant and extremely alkaliphilic bacteria.
Figures

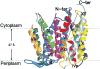
Similar articles
-
Multidrug resistance protein MdtM adds to the repertoire of antiporters involved in alkaline pH homeostasis in Escherichia coli.BMC Microbiol. 2013 May 23;13:113. doi: 10.1186/1471-2180-13-113. BMC Microbiol. 2013. PMID: 23701827 Free PMC article.
-
Sodium-Proton (Na(+)/H(+)) Antiporters: Properties and Roles in Health and Disease.Met Ions Life Sci. 2016;16:391-458. doi: 10.1007/978-3-319-21756-7_12. Met Ions Life Sci. 2016. PMID: 26860308
-
Escherichia coli YqjA, a Member of the Conserved DedA/Tvp38 Membrane Protein Family, Is a Putative Osmosensing Transporter Required for Growth at Alkaline pH.J Bacteriol. 2015 Jul;197(14):2292-300. doi: 10.1128/JB.00175-15. Epub 2015 Apr 27. J Bacteriol. 2015. PMID: 25917916 Free PMC article.
-
pH homeostasis and ATP synthesis: studies of two processes that necessitate inward proton translocation in extremely alkaliphilic Bacillus species.Extremophiles. 1998 Aug;2(3):217-22. doi: 10.1007/s007920050063. Extremophiles. 1998. PMID: 9783168 Review.
-
NhaA of Escherichia coli, as a model of a pH-regulated Na+/H+antiporter.Biochim Biophys Acta. 2004 Jul 23;1658(1-2):2-13. doi: 10.1016/j.bbabio.2004.04.018. Biochim Biophys Acta. 2004. PMID: 15282168 Review.
Cited by
-
Generation of high current densities by pure cultures of anode-respiring Geoalkalibacter spp. under alkaline and saline conditions in microbial electrochemical cells.mBio. 2013 Apr 30;4(3):e00144-13. doi: 10.1128/mBio.00144-13. mBio. 2013. PMID: 23631915 Free PMC article.
-
In vitro cytocompatibility and antibacterial studies on biodegradable Zn alloys supplemented by a critical assessment of direct contact cytotoxicity assay.J Biomed Mater Res B Appl Biomater. 2023 Feb;111(2):241-260. doi: 10.1002/jbm.b.35147. Epub 2022 Aug 26. J Biomed Mater Res B Appl Biomater. 2023. PMID: 36054531 Free PMC article.
-
Antibiotic Bactericidal Activity Is Countered by Maintaining pH Homeostasis in Mycobacterium smegmatis.mSphere. 2016 Aug 24;1(4):e00176-16. doi: 10.1128/mSphere.00176-16. eCollection 2016 Jul-Aug. mSphere. 2016. PMID: 27579369 Free PMC article.
-
pH-induced change in cell susceptibility to butanol in a high butanol-tolerant bacterium, Enterococcus faecalis strain CM4A.Biotechnol Biofuels. 2015 Apr 17;8:69. doi: 10.1186/s13068-015-0251-x. eCollection 2015. Biotechnol Biofuels. 2015. PMID: 25904984 Free PMC article.
-
Halotolerant cyanobacterium Aphanothece halophytica contains a betaine transporter active at alkaline pH and high salinity.Appl Environ Microbiol. 2006 Sep;72(9):6018-26. doi: 10.1128/AEM.00733-06. Appl Environ Microbiol. 2006. PMID: 16957224 Free PMC article.
References
-
- Padan E, Zilberstein D, Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976;63:533–541. - PubMed
-
- Padan E, Zilberstein D, Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981;650:151–166. - PubMed
-
- Collins MD, Lawson PA, Willems A, Cordoba JJ, Fernandez-Garayzabal J, Garcia P, Cai J, Hippe H, Farrow JA. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

