Neuronal activation by GPI-linked neuroligin-1 displayed in synthetic lipid bilayer membranes
- PMID: 16262338
- PMCID: PMC1448170
- DOI: 10.1021/la051243d
Neuronal activation by GPI-linked neuroligin-1 displayed in synthetic lipid bilayer membranes
Abstract
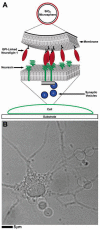
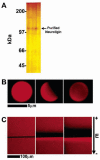
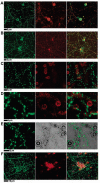
We have characterized, in vitro, interactions between hippocampal neuronal cells and silica microbeads coated with synthetic, fluid, lipid bilayer membranes containing the glycosylphosphatidyl inositol (GPI)-linked extracellular domain of the postsynaptic membrane protein neuroligin-1. These bilayer-neuroligin-1 beads activated neuronal cells to form presynaptic nerve terminals at the point of contact in a manner similar to that observed for live PC12 cells, ectopically expressing the full length neuroligin-1. The synthetic membranes exhibited biological activity at neuroligin-1 densities of approximately 1 to 6 proteins/microm(2). Polyolycarbonate beads with neuroligin-1 covalently attached to the surface failed to activate neurons despite the fact that neuroligin-1 binding activity is preserved. This implies that a lipid membrane environment is likely to be essential for neuroligin-1 activity. This technique allows the study of isolated proteins in an environment that has physical properties resembling those of a cell surface; proteins can diffuse freely within the membrane, retain their in vivo orientations, and are in a nondenatured state. In addition, the synthetic membrane environment affords control over both lipid and protein composition. This technology is easily implemented and can be applied to a wide variety of cellular studies.
Figures


Similar articles
-
Neuronal synapse interaction reconstituted between live cells and supported lipid bilayers.Nat Chem Biol. 2005 Oct;1(5):283-9. doi: 10.1038/nchembio737. Epub 2005 Sep 18. Nat Chem Biol. 2005. PMID: 16408058 Free PMC article.
-
Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules.J Biol Chem. 1997 Oct 10;272(41):26032-9. doi: 10.1074/jbc.272.41.26032. J Biol Chem. 1997. PMID: 9325340
-
A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins.Neuron. 2005 Oct 20;48(2):229-36. doi: 10.1016/j.neuron.2005.08.026. Neuron. 2005. PMID: 16242404
-
Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses.Proc Natl Acad Sci U S A. 1999 Feb 2;96(3):1100-5. doi: 10.1073/pnas.96.3.1100. Proc Natl Acad Sci U S A. 1999. PMID: 9927700 Free PMC article.
-
Assembly of synapses: biomimetic assays to control neurexin/neuroligin interactions at the neuronal surface.Curr Protoc Neurosci. 2013 Jul;Chapter 2:Unit 2.19. doi: 10.1002/0471142301.ns0219s64. Curr Protoc Neurosci. 2013. PMID: 23853109
Cited by
-
Neuroligin-1 performs neurexin-dependent and neurexin-independent functions in synapse validation.EMBO J. 2009 Oct 21;28(20):3244-55. doi: 10.1038/emboj.2009.249. Epub 2009 Sep 3. EMBO J. 2009. PMID: 19730411 Free PMC article.
-
Direct quantitative detection of Doc2b-induced hemifusion in optically trapped membranes.Nat Commun. 2015 Sep 23;6:8387. doi: 10.1038/ncomms9387. Nat Commun. 2015. PMID: 26395669 Free PMC article.
-
Streamlining the interface between electronics and neural systems for bidirectional electrochemical communication.Chem Sci. 2023 Apr 14;14(17):4463-4479. doi: 10.1039/d3sc00338h. eCollection 2023 May 3. Chem Sci. 2023. PMID: 37152246 Free PMC article. Review.
-
Neurexin/neuroligin interaction kinetics characterized by counting single cell-surface attached quantum dots.Biophys J. 2009 Jul 22;97(2):480-9. doi: 10.1016/j.bpj.2009.04.044. Biophys J. 2009. PMID: 19619462 Free PMC article.
-
Artificial Lipid Membranes: Past, Present, and Future.Membranes (Basel). 2017 Jul 26;7(3):38. doi: 10.3390/membranes7030038. Membranes (Basel). 2017. PMID: 28933723 Free PMC article. Review.
References
-
- Maheswari G, et al. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Science. 2000;113:1677–1686. - PubMed
-
- Mammen M, Choi S-K, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 1998;37:2754–2794. - PubMed
-
- Cochran JR, Cameron TO, Stern LJ. The relationship of MHC-peptide binding and T cell activation using chemically defined MHC class II oligomers. Immunity. 2000;12:241–250. - PubMed
-
- Kiessling LL, Gestwicki JE, Strong L. Synthetic multivalent ligands in the exploration of cell-surface interactions. Curr. Opin. Chem. Biol. 2000;4:696–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

