Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis
- PMID: 16260496
- PMCID: PMC2171270
- DOI: 10.1083/jcb.200504091
Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis
Abstract
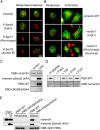
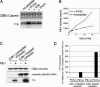
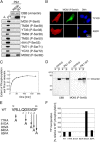
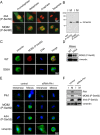
Several kinases phosphorylate vimentin, the most common intermediate filament protein, in mitosis. Aurora-B and Rho-kinase regulate vimentin filament separation through the cleavage furrow-specific vimentin phosphorylation. Cdk1 also phosphorylates vimentin from prometaphase to metaphase, but its significance has remained unknown. Here we demonstrated a direct interaction between Plk1 and vimentin-Ser55 phosphorylated by Cdk1, an event that led to Plk1 activation and further vimentin phosphorylation. Plk1 phosphorylated vimentin at approximately 1 mol phosphate/mol substrate, which partly inhibited its filament forming ability, in vitro. Plk1 induced the phosphorylation of vimentin-Ser82, which was elevated from metaphase and maintained until the end of mitosis. This elevation followed the Cdk1-induced vimentin-Ser55 phosphorylation, and was impaired by Plk1 depletion. Mutational analyses revealed that Plk1-induced vimentin-Ser82 phosphorylation plays an important role in vimentin filaments segregation, coordinately with Rho-kinase and Aurora-B. Taken together, these results indicated a novel mechanism that Cdk1 regulated mitotic vimentin phosphorylation via not only a direct enzyme reaction but also Plk1 recruitment to vimentin.
Figures
Similar articles
-
Choice of Plk1 docking partners during mitosis and cytokinesis is controlled by the activation state of Cdk1.Nat Cell Biol. 2007 Apr;9(4):436-44. doi: 10.1038/ncb1557. Epub 2007 Mar 11. Nat Cell Biol. 2007. PMID: 17351640
-
Sequential phosphorylation of Nedd1 by Cdk1 and Plk1 is required for targeting of the gammaTuRC to the centrosome.J Cell Sci. 2009 Jul 1;122(Pt 13):2240-51. doi: 10.1242/jcs.042747. Epub 2009 Jun 9. J Cell Sci. 2009. PMID: 19509060
-
Aurora-B and Rho-kinase/ROCK, the two cleavage furrow kinases, independently regulate the progression of cytokinesis: possible existence of a novel cleavage furrow kinase phosphorylates ezrin/radixin/moesin (ERM).Genes Cells. 2005 Feb;10(2):127-37. doi: 10.1111/j.1365-2443.2005.00824.x. Genes Cells. 2005. PMID: 15676024
-
Cytokinesis and cancer: Polo loves ROCK'n' Rho(A).J Genet Genomics. 2010 Mar;37(3):159-72. doi: 10.1016/S1673-8527(09)60034-5. J Genet Genomics. 2010. PMID: 20347825 Review.
-
Regulatory mechanisms and functions of intermediate filaments: a study using site- and phosphorylation state-specific antibodies.Cancer Sci. 2006 Mar;97(3):167-74. doi: 10.1111/j.1349-7006.2006.00161.x. Cancer Sci. 2006. PMID: 16542212 Free PMC article. Review.
Cited by
-
Wee1 is required to sustain ATR/Chk1 signaling upon replicative stress.Oncotarget. 2015 May 30;6(15):13072-87. doi: 10.18632/oncotarget.3865. Oncotarget. 2015. PMID: 25965828 Free PMC article.
-
Role of p47(phox) in regulating Cdc42GAP, vimentin, and contraction in smooth muscle cells.Am J Physiol Cell Physiol. 2009 Dec;297(6):C1424-33. doi: 10.1152/ajpcell.00324.2009. Epub 2009 Oct 7. Am J Physiol Cell Physiol. 2009. PMID: 19812368 Free PMC article.
-
Non-canonical cMet regulation by vimentin mediates Plk1 inhibitor-induced apoptosis.EMBO Mol Med. 2019 May;11(5):e9960. doi: 10.15252/emmm.201809960. EMBO Mol Med. 2019. PMID: 31040125 Free PMC article.
-
CircGLIS3 promotes gastric cancer progression by regulating the miR-1343-3p/PGK1 pathway and inhibiting vimentin phosphorylation.J Transl Med. 2024 Mar 8;22(1):251. doi: 10.1186/s12967-023-04625-2. J Transl Med. 2024. PMID: 38459513 Free PMC article.
-
O-GlcNAc: A Sweetheart of the Cell Cycle and DNA Damage Response.Front Endocrinol (Lausanne). 2018 Jul 30;9:415. doi: 10.3389/fendo.2018.00415. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30105004 Free PMC article. Review.
References
-
- Barr, F.A., H.H. Silljé, and E.A. Nigg. 2004. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 5:429–440. - PubMed
-
- Carmena, M., and W.C. Earnshaw. 2003. The cellular geography of aurora kinases. Nat. Rev. Mol. Cell Biol. 4:842–854. - PubMed
-
- Chang, L., and R.D. Goldman. 2004. Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 5:601–613. - PubMed
-
- Chou, Y.H., K.L. Ngai, and R. Goldman. 1991. The regulation of intermediate filament reorganization in mitosis. p34cdc2 phosphorylates vimentin at a unique N-terminal site. J. Biol. Chem. 266:7325–7328. - PubMed
-
- Elia, A.E., L.C. Cantley, and M.B. Yaffe. 2003. a. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science. 299:1228–1231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

