TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo
- PMID: 16260493
- PMCID: PMC1283960
- DOI: 10.1101/gad.1360605
TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo
Abstract
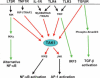
TGF-beta-activated kinase 1 (TAK1), a member of the MAPKKK family, is thought to be a key modulator of the inducible transcription factors NF-kappaB and AP-1 and, therefore, plays a crucial role in regulating the genes that mediate inflammation. Although in vitro biochemical studies have revealed the existence of a TAK1 complex, which includes TAK1 and the adapter proteins TAB1 and TAB2, it remains unclear which members of this complex are essential for signaling. To analyze the function of TAK1 in vivo, we have deleted the Tak1 gene in mice, with the resulting phenotype being early embryonic lethality. Using embryonic fibroblasts lacking TAK1, TAB1, or TAB2, we have found that TNFR1, IL-1R, TLR3, and TLR4-mediated NF-kappaB and AP-1 activation are severely impaired in Tak1(m/m) cells, but they are normal in Tab1(-/-) and Tab2(-/-) cells. In addition, Tak1(m/m) cells are highly sensitive to TNF-induced apoptosis. TAK1 mediates IKK activation in TNF-alpha and IL-1 signaling pathways, where it functions downstream of RIP1-TRAF2 and MyD88-IRAK1-TRAF6, respectively. However, TAK1 is not required for NF-kappaB activation through the alternative pathway following LT-beta signaling. In the TGF-beta signaling pathway, TAK1 deletion leads to impaired NF-kappaB and c-Jun N-terminal kinase (JNK) activation without impacting Smad2 activation or TGF-beta-induced gene expression. Therefore, our studies suggests that TAK1 acts as an upstream activating kinase for IKKbeta and JNK, but not IKKalpha, revealing an unexpectedly specific role of TAK1 in inflammatory signaling pathways.
Figures



Similar articles
-
Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta.Proc Natl Acad Sci U S A. 2004 Mar 9;101(10):3533-8. doi: 10.1073/pnas.0308496101. Epub 2004 Feb 24. Proc Natl Acad Sci U S A. 2004. PMID: 14982987 Free PMC article.
-
Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells.Immunobiology. 2013 Dec;218(12):1452-67. doi: 10.1016/j.imbio.2013.04.019. Epub 2013 May 9. Immunobiology. 2013. PMID: 23735482
-
Phosphoinositide-dependent kinase-1 inhibits TRAF6 ubiquitination by interrupting the formation of TAK1-TAB2 complex in TLR4 signaling.Cell Signal. 2015 Dec;27(12):2524-33. doi: 10.1016/j.cellsig.2015.09.018. Epub 2015 Sep 30. Cell Signal. 2015. PMID: 26432169
-
Post-Translational Modifications of the TAK1-TAB Complex.Int J Mol Sci. 2017 Jan 19;18(1):205. doi: 10.3390/ijms18010205. Int J Mol Sci. 2017. PMID: 28106845 Free PMC article. Review.
-
TAK1-TABs Complex: A Central Signalosome in Inflammatory Responses.Front Immunol. 2021 Jan 5;11:608976. doi: 10.3389/fimmu.2020.608976. eCollection 2020. Front Immunol. 2021. PMID: 33469458 Free PMC article. Review.
Cited by
-
Role of TGFβ in regulation of the tumor microenvironment and drug delivery (review).Int J Oncol. 2015 Mar;46(3):933-43. doi: 10.3892/ijo.2015.2816. Epub 2015 Jan 7. Int J Oncol. 2015. PMID: 25573346 Free PMC article. Review.
-
Cardiomyocyte-specific knockout of ADAM17 alleviates doxorubicin-induced cardiomyopathy via inhibiting TNFα-TRAF3-TAK1-MAPK axis.Signal Transduct Target Ther. 2024 Oct 16;9(1):273. doi: 10.1038/s41392-024-01977-z. Signal Transduct Target Ther. 2024. PMID: 39406701 Free PMC article.
-
Identification and functional characterization of novel phosphorylation sites in TAK1-binding protein (TAB) 1.PLoS One. 2011;6(12):e29256. doi: 10.1371/journal.pone.0029256. Epub 2011 Dec 22. PLoS One. 2011. PMID: 22216226 Free PMC article.
-
Aberrantly activated TAK1 links neuroinflammation and neuronal loss in Alzheimer's disease mouse models.J Cell Sci. 2023 Mar 15;136(6):jcs260102. doi: 10.1242/jcs.260102. Epub 2023 Mar 13. J Cell Sci. 2023. PMID: 36912451 Free PMC article.
-
Transforming growth factor-beta-activated kinase 1 is an essential regulator of myogenic differentiation.J Biol Chem. 2010 Feb 26;285(9):6401-11. doi: 10.1074/jbc.M109.064063. Epub 2009 Dec 27. J Biol Chem. 2010. PMID: 20037161 Free PMC article.
References
-
- Baud V. and Karin, M. 2001. Signal transduction by tumor necrosis factor and its relatives. Trends Cell. Biol. 11: 372–377. - PubMed
-
- Beg A.A. and Baltimore, D. 1996. An essential role for NF-κBin preventing TNF-α-induced cell death. Science 274: 782–784. - PubMed
-
- Beg A.A., Sha, W.C., Bronson, R.T., Ghosh, S., and Baltimore, D. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κ B. Nature 376: 167–170. - PubMed
-
- Bonizzi G. and Karin, M. 2004. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends. Immunol. 25: 280–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
