Hair cycle and wound healing in mice with a keratinocyte-restricted deletion of FAK
- PMID: 16247468
- PMCID: PMC2710133
- DOI: 10.1038/sj.onc.1209130
Hair cycle and wound healing in mice with a keratinocyte-restricted deletion of FAK
Abstract
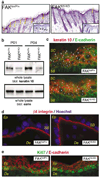
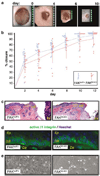
Focal adhesion kinase (FAK) is a critical component in transducing signals downstream of both integrins and growth factor receptors. To determine how the loss of FAK affects the epidermis in vivo, we have generated a mouse model with a keratinocyte-restricted deletion of fak (FAKK5 KO mice). FAK(K5 KO) mice displayed three major phenotypes--irregularities of hair cycle, sebaceous glands hypoplasia, and a thinner epidermis--pointing to defects in the proliferative capacity of multipotent stem cells found in the bulge. FAK-null keratinocytes in conventional primary culture undergo massive apoptosis hindering further analyses, whereas the defects observed in vivo do not shorten the mouse lifespan. These results suggest that the structure and the signaling environment of the native tissue may overcome the lack of signaling through FAK. Our findings point to the importance of in vivo and three-dimensional in vitro models in analyses of cell migration, proliferation, and survival. Surprisingly, the difference between FAKloxP/+ and FAKK5 KO mice in wound closure was not statistically significant, suggesting that in vivo loss of FAK does not affect migration/proliferation of basal keratinocytes in the same way as it affects multipotent stem cells of the skin.
Figures



Similar articles
-
Loss of keratinocyte focal adhesion kinase stimulates dermal proteolysis through upregulation of MMP9 in wound healing.Ann Surg. 2014 Dec;260(6):1138-46. doi: 10.1097/SLA.0000000000000219. Ann Surg. 2014. PMID: 25389925
-
Delayed wound healing in keratin 6a knockout mice.Mol Cell Biol. 2000 Jul;20(14):5248-55. doi: 10.1128/MCB.20.14.5248-5255.2000. Mol Cell Biol. 2000. PMID: 10866680 Free PMC article.
-
Alpha 3 beta 1 integrin promotes keratinocyte cell survival through activation of a MEK/ERK signaling pathway.J Cell Sci. 2004 Aug 15;117(Pt 18):4043-54. doi: 10.1242/jcs.01277. Epub 2004 Jul 27. J Cell Sci. 2004. PMID: 15280429
-
Focal adhesion kinase controls pH-dependent epidermal barrier homeostasis by regulating actin-directed Na+/H+ exchanger 1 plasma membrane localization.Am J Pathol. 2007 Jun;170(6):2055-67. doi: 10.2353/ajpath.2007.061277. Am J Pathol. 2007. PMID: 17525272 Free PMC article.
-
Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression.Genes Dev. 2004 Dec 15;18(24):2998-3003. doi: 10.1101/gad.316304. Genes Dev. 2004. PMID: 15601818 Free PMC article.
Cited by
-
Tissue Mechanics in Haired Murine Skin: Potential Implications for Skin Aging.Front Cell Dev Biol. 2021 Feb 19;9:635340. doi: 10.3389/fcell.2021.635340. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33681217 Free PMC article.
-
Keratin 5-Cre-driven excision of nonmuscle myosin IIA in early embryo trophectoderm leads to placenta defects and embryonic lethality.Dev Biol. 2013 Oct 1;382(1):136-48. doi: 10.1016/j.ydbio.2013.07.017. Epub 2013 Jul 30. Dev Biol. 2013. PMID: 23911870 Free PMC article.
-
FAK regulates cardiomyocyte survival following ischemia/reperfusion.J Mol Cell Cardiol. 2009 Feb;46(2):241-8. doi: 10.1016/j.yjmcc.2008.10.017. Epub 2008 Nov 5. J Mol Cell Cardiol. 2009. PMID: 19028502 Free PMC article.
-
FGF receptors 1 and 2 are key regulators of keratinocyte migration in vitro and in wounded skin.J Cell Sci. 2012 Dec 1;125(Pt 23):5690-701. doi: 10.1242/jcs.108167. Epub 2012 Sep 19. J Cell Sci. 2012. PMID: 22992463 Free PMC article.
-
Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells.Exp Cell Res. 2007 Jan 1;313(1):22-37. doi: 10.1016/j.yexcr.2006.09.013. Epub 2006 Sep 22. Exp Cell Res. 2007. PMID: 17081517 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

