Enhanced tolerance to environmental stress in transgenic plants expressing the transcriptional coactivator multiprotein bridging factor 1c
- PMID: 16244138
- PMCID: PMC1283768
- DOI: 10.1104/pp.105.070110
Enhanced tolerance to environmental stress in transgenic plants expressing the transcriptional coactivator multiprotein bridging factor 1c
Abstract
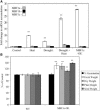
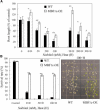
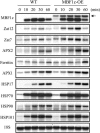
Abiotic stresses cause extensive losses to agricultural production worldwide. Acclimation of plants to abiotic conditions such as drought, salinity, or heat is mediated by a complex network of transcription factors and other regulatory genes that control multiple defense enzymes, proteins, and pathways. Associated with the activity of different transcription factors are transcriptional coactivators that enhance their binding to the basal transcription machinery. Although the importance of stress-response transcription factors was demonstrated in transgenic plants, little is known about the function of transcriptional coactivators associated with abiotic stresses. Here, we report that constitutive expression of the stress-response transcriptional coactivator multiprotein bridging factor 1c (MBF1c) in Arabidopsis (Arabidopsis thaliana) enhances the tolerance of transgenic plants to bacterial infection, heat, and osmotic stress. Moreover, the enhanced tolerance of transgenic plants to osmotic and heat stress was maintained even when these two stresses were combined. The expression of MBF1c in transgenic plants augmented the accumulation of a number of defense transcripts in response to heat stress. Transcriptome profiling and inhibitor studies suggest that MBF1c expression enhances the tolerance of transgenic plants to heat and osmotic stress by partially activating, or perturbing, the ethylene-response signal transduction pathway. Present findings suggest that MBF1 proteins could be used to enhance the tolerance of plants to different abiotic stresses.
Figures
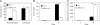
Similar articles
-
Identification of the MBF1 heat-response regulon of Arabidopsis thaliana.Plant J. 2011 Jun;66(5):844-51. doi: 10.1111/j.1365-313X.2011.04550.x. Epub 2011 Apr 4. Plant J. 2011. PMID: 21457365 Free PMC article.
-
The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana.J Biol Chem. 2008 Apr 4;283(14):9269-75. doi: 10.1074/jbc.M709187200. Epub 2008 Jan 17. J Biol Chem. 2008. PMID: 18201973
-
Vascular plant one-zinc-finger protein 1/2 transcription factors regulate abiotic and biotic stress responses in Arabidopsis.Plant J. 2013 Mar;73(5):761-75. doi: 10.1111/tpj.12069. Epub 2013 Feb 12. Plant J. 2013. PMID: 23167462
-
Generating high temperature tolerant transgenic plants: Achievements and challenges.Plant Sci. 2013 May;205-206:38-47. doi: 10.1016/j.plantsci.2013.01.005. Epub 2013 Jan 30. Plant Sci. 2013. PMID: 23498861 Review.
-
The role of transcriptional coactivator ADA2b in Arabidopsis abiotic stress responses.Plant Signal Behav. 2011 Oct;6(10):1475-8. doi: 10.4161/psb.6.10.17695. Epub 2011 Oct 1. Plant Signal Behav. 2011. PMID: 21897124 Free PMC article. Review.
Cited by
-
STRESS RESPONSE SUPPRESSOR1 and STRESS RESPONSE SUPPRESSOR2, two DEAD-box RNA helicases that attenuate Arabidopsis responses to multiple abiotic stresses.Plant Physiol. 2007 Nov;145(3):814-30. doi: 10.1104/pp.107.099895. Epub 2007 Jun 7. Plant Physiol. 2007. PMID: 17556511 Free PMC article.
-
Glutathione modulates the expression of heat shock proteins via the transcription factors BZIP10 and MYB21 in Arabidopsis.J Exp Bot. 2018 Jun 27;69(15):3729-3743. doi: 10.1093/jxb/ery166. J Exp Bot. 2018. PMID: 29722824 Free PMC article.
-
On the role of ethylene, auxin and a GOLVEN-like peptide hormone in the regulation of peach ripening.BMC Plant Biol. 2016 Feb 11;16:44. doi: 10.1186/s12870-016-0730-7. BMC Plant Biol. 2016. PMID: 26863869 Free PMC article.
-
Arabidopsis thaliana-Myzus persicae interaction: shaping the understanding of plant defense against phloem-feeding aphids.Front Plant Sci. 2013 Jul 1;4:213. doi: 10.3389/fpls.2013.00213. eCollection 2013. Front Plant Sci. 2013. PMID: 23847627 Free PMC article.
-
A CAM-Related NF-YB Transcription Factor Enhances Multiple Abiotic Stress Tolerance in Arabidopsis.Int J Mol Sci. 2024 Jun 28;25(13):7107. doi: 10.3390/ijms25137107. Int J Mol Sci. 2024. PMID: 39000218 Free PMC article.
References
-
- Boyer JS (1982) Plant productivity and environment. Science 218: 443–448 - PubMed
-
- Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stresses. In W Gruissem, B Buchannan, R Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp 1158–1249
-
- Brendel C, Gelman L, Auwerx J (2002) Multiprotein bridging factor-1 (MBF-1) is a cofactor for nuclear receptors that regulate lipid metabolism. Mol Endocrinol 16: 1367–1377 - PubMed
-
- Busk PK, Wulf-Andersen L, Strom CC, Enevoldsen M, Thirstrup K, Haunso S, Sheikh SP (2003) Multiprotein bridging factor 1 cooperates with c-Jun and is necessary for cardiac hypertrophy in vitro. Exp Cell Res 286: 102–114 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

