Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system
- PMID: 16227210
- PMCID: PMC2586128
- DOI: 10.1074/jbc.M504963200
Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system
Abstract
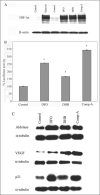
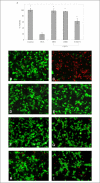
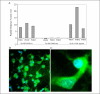
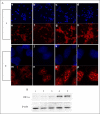
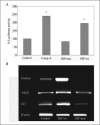
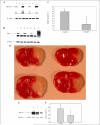
Hypoxia-inducible factor (HIF) prolyl 4-hydroxylases are a family of iron- and 2-oxoglutarate-dependent dioxygenases that negatively regulate the stability of several proteins that have established roles in adaptation to hypoxic or oxidative stress. These proteins include the transcriptional activators HIF-1alpha and HIF-2alpha. The ability of the inhibitors of HIF prolyl 4-hydroxylases to stabilize proteins involved in adaptation in neurons and to prevent neuronal injury remains unclear. We reported that structurally diverse low molecular weight or peptide inhibitors of the HIF prolyl 4-hydroxylases stabilize HIF-1alpha and up-regulate HIF-dependent target genes (e.g. enolase, p21(waf1/cip1), vascular endothelial growth factor, or erythropoietin) in embryonic cortical neurons in vitro or in adult rat brains in vivo. We also showed that structurally diverse HIF prolyl 4-hydroxylase inhibitors prevent oxidative death in vitro and ischemic injury in vivo. Taken together these findings identified low molecular weight and peptide HIF prolyl 4-hydroxylase inhibitors as novel neurological therapeutics for stroke as well as other diseases associated with oxidative stress.
Figures

Similar articles
-
Increased prolyl 4-hydroxylase expression and differential regulation of hypoxia-inducible factors in the aged rat brain.Am J Physiol Regul Integr Comp Physiol. 2009 Jul;297(1):R158-65. doi: 10.1152/ajpregu.90829.2008. Epub 2009 May 6. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19420289 Free PMC article.
-
Selective inhibition of hypoxia-inducible factor (HIF) prolyl-hydroxylase 1 mediates neuroprotection against normoxic oxidative death via HIF- and CREB-independent pathways.J Neurosci. 2009 Jul 8;29(27):8828-38. doi: 10.1523/JNEUROSCI.1779-09.2009. J Neurosci. 2009. PMID: 19587290 Free PMC article.
-
Short-term effects of pharmacologic HIF stabilization on vasoactive and cytotrophic factors in developing mouse brain.Brain Res. 2009 Jul 14;1280:43-51. doi: 10.1016/j.brainres.2009.05.023. Epub 2009 May 18. Brain Res. 2009. PMID: 19450570
-
HIF-prolyl hydroxylases and cardiovascular diseases.Toxicol Mech Methods. 2012 Jun;22(5):347-58. doi: 10.3109/15376516.2012.673088. Toxicol Mech Methods. 2012. PMID: 22424133 Review.
-
Hypoxia inducible factor prolyl 4-hydroxylase enzymes: center stage in the battle against hypoxia, metabolic compromise and oxidative stress.Neurochem Res. 2007 Apr-May;32(4-5):931-46. doi: 10.1007/s11064-006-9268-7. Epub 2007 Mar 7. Neurochem Res. 2007. PMID: 17342411 Free PMC article. Review.
Cited by
-
Cell type-specific dependency on the PI3K/Akt signaling pathway for the endogenous Epo and VEGF induction by baicalein in neurons versus astrocytes.PLoS One. 2013 Jul 19;8(7):e69019. doi: 10.1371/journal.pone.0069019. Print 2013. PLoS One. 2013. PMID: 23904909 Free PMC article.
-
Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis.Nat Biotechnol. 2010 Mar;28(3):249-55. doi: 10.1038/nbt.1606. Epub 2010 Feb 14. Nat Biotechnol. 2010. PMID: 20160716 Free PMC article.
-
Antioxidants, HIF prolyl hydroxylase inhibitors or short interfering RNAs to BNIP3 or PUMA, can prevent prodeath effects of the transcriptional activator, HIF-1alpha, in a mouse hippocampal neuronal line.Antioxid Redox Signal. 2008 Dec;10(12):1989-98. doi: 10.1089/ars.2008.2039. Antioxid Redox Signal. 2008. PMID: 18774900 Free PMC article.
-
Prodeath or prosurvival: two facets of hypoxia inducible factor-1 in perinatal brain injury.Exp Neurol. 2009 Mar;216(1):7-15. doi: 10.1016/j.expneurol.2008.10.016. Epub 2008 Nov 11. Exp Neurol. 2009. PMID: 19041643 Free PMC article. Review.
-
Noxious Iron-Calcium Connections in Neurodegeneration.Front Neurosci. 2019 Feb 12;13:48. doi: 10.3389/fnins.2019.00048. eCollection 2019. Front Neurosci. 2019. PMID: 30809110 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

