Endogenous human cytomegalovirus gB is presented efficiently by MHC class II molecules to CD4+ CTL
- PMID: 16216889
- PMCID: PMC2213219
- DOI: 10.1084/jem.20050162
Endogenous human cytomegalovirus gB is presented efficiently by MHC class II molecules to CD4+ CTL
Abstract
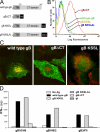
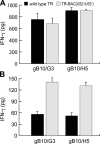
Human cytomegalovirus (HCMV) infects endothelial, epithelial, and glial cells in vivo. These cells can express MHC class II proteins, but are unlikely to play important roles in priming host immunity. Instead, it seems that class II presentation of endogenous HCMV antigens in these cells allows recognition of virus infection. We characterized class II presentation of HCMV glycoprotein B (gB), a membrane protein that accumulates extensively in endosomes during virus assembly. Human CD4+ T cells specific for gB were both highly abundant in blood and cytolytic in vivo. gB-specific CD4+ T cell clones recognized gB that was expressed in glial, endothelial, and epithelial cells, but not exogenous gB that was fed to these cells. Glial cells efficiently presented extremely low levels of endogenous gB--expressed by adenovirus vectors or after HCMV infection--and stimulated CD4+ T cells better than DCs that were incubated with exogenous gB. Presentation of endogenous gB required sorting of gB to endosomal compartments and processing by acidic proteases. Although presentation of cellular proteins that traffic into endosomes is well known, our observations demonstrate for the first time that a viral protein sorted to endosomes is presented exceptionally well, and can promote CD4+ T cell recognition and killing of biologically important host cells.
Figures

Similar articles
-
Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells.Nat Med. 1999 Sep;5(9):1039-43. doi: 10.1038/12478. Nat Med. 1999. PMID: 10470081
-
Cytotoxic T cell immunity to human cytomegalovirus glycoprotein B.J Med Virol. 1996 Jun;49(2):124-31. doi: 10.1002/(SICI)1096-9071(199606)49:2<124::AID-JMV9>3.0.CO;2-7. J Med Virol. 1996. PMID: 8991935
-
Inhibition of HLA-DR assembly, transport, and loading by human cytomegalovirus glycoprotein US3: a novel mechanism for evading major histocompatibility complex class II antigen presentation.J Virol. 2002 Nov;76(21):10929-41. doi: 10.1128/jvi.76.21.10929-10941.2002. J Virol. 2002. PMID: 12368336 Free PMC article.
-
Inhibition of the MHC class II antigen presentation pathway by human cytomegalovirus.Curr Top Microbiol Immunol. 2002;269:101-15. doi: 10.1007/978-3-642-59421-2_7. Curr Top Microbiol Immunol. 2002. PMID: 12224504 Review.
-
Autophagy in MHC class II antigen processing.Curr Opin Immunol. 2007 Feb;19(1):87-92. doi: 10.1016/j.coi.2006.11.009. Epub 2006 Nov 28. Curr Opin Immunol. 2007. PMID: 17129719 Review.
Cited by
-
Cytotoxic CD4 T cells in antiviral immunity.J Biomed Biotechnol. 2011;2011:954602. doi: 10.1155/2011/954602. Epub 2011 Nov 22. J Biomed Biotechnol. 2011. PMID: 22174559 Free PMC article. Review.
-
Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts.J Exp Med. 2010 Mar 15;207(3):637-50. doi: 10.1084/jem.20091918. Epub 2010 Feb 15. J Exp Med. 2010. PMID: 20156971 Free PMC article.
-
Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans.J Virol. 2008 Dec;82(23):11837-50. doi: 10.1128/JVI.01623-08. Epub 2008 Sep 24. J Virol. 2008. PMID: 18815310 Free PMC article.
-
The elucidation of non-classical MHC class II antigen processing through the study of viral antigens.Curr Opin Virol. 2017 Feb;22:71-76. doi: 10.1016/j.coviro.2016.11.009. Epub 2017 Jan 12. Curr Opin Virol. 2017. PMID: 28081485 Free PMC article. Review.
-
The Cellular Localization of Human Cytomegalovirus Glycoprotein Expression Greatly Influences the Frequency and Functional Phenotype of Specific CD4+ T Cell Responses.J Immunol. 2015 Oct 15;195(8):3803-15. doi: 10.4049/jimmunol.1500696. Epub 2015 Sep 11. J Immunol. 2015. PMID: 26363059 Free PMC article. Clinical Trial.
References
-
- Rudensky, A., P. Preston-Hurlburt, S.C. Hong, A. Barlow, and C.A. Janeway Jr. 1991. Sequence analysis of peptides bound to MHC class II molecules. Nature. 353:622–627. - PubMed
-
- Nimmerjahn, F., S. Milosevic, U. Behrends, E.M. Jaffee, D.M. Pardoll, G.W. Bornkamm, and J. Mautner. 2003. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur. J. Immunol. 33:1250–1259. - PubMed
-
- Dani, A., A. Chaudhry, P. Mukherjee, D. Rajagopal, S. Bhatia, A. George, V. Bal, S. Rath, and S. Mayor. 2004. The pathway for MHCII-mediated presentation of endogenous proteins involves peptide transport to the endo-lysosomal compartment. J. Cell Sci. 117:4219–4230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

