Obstacles and advances in SARS vaccine development
- PMID: 16191455
- PMCID: PMC7115537
- DOI: 10.1016/j.vaccine.2005.08.102
Obstacles and advances in SARS vaccine development
Abstract
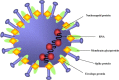
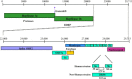
The emergence of the severe acute respiratory syndrome (SARS) that resulted in a pandemic in 2003 spurred a flurry of interest in the development of vaccines to prevent and treat the potentially deadly viral infection. Researchers around the world pooled their scientific resources and shared early data in an unprecedented manner in light of the impending public health crisis. There are still large gaps in knowledge about the pathogenesis of this virus. While significant advances have been made in the development of animal models, the practicality of their use may be hampered by a lack of pathological similarity with human disease. Described here are issues related to progress in vaccine development and the obstacles that lie ahead for both researchers and regulatory agencies.
Figures
Similar articles
-
Severe acute respiratory syndrome: vaccine on the way.Chin Med J (Engl). 2005 Sep 5;118(17):1468-76. Chin Med J (Engl). 2005. PMID: 16157050 Review. No abstract available.
-
SARS vaccines: where are we?Expert Rev Vaccines. 2009 Jul;8(7):887-98. doi: 10.1586/erv.09.43. Expert Rev Vaccines. 2009. PMID: 19538115 Free PMC article. Review.
-
Vaccines for COVID-19: The current state of play.Paediatr Respir Rev. 2020 Sep;35:43-49. doi: 10.1016/j.prrv.2020.06.010. Epub 2020 Jun 18. Paediatr Respir Rev. 2020. PMID: 32653463 Free PMC article. Review.
-
Vaccines to prevent severe acute respiratory syndrome coronavirus-induced disease.Virus Res. 2008 Apr;133(1):45-62. doi: 10.1016/j.virusres.2007.01.021. Epub 2007 Apr 9. Virus Res. 2008. PMID: 17416434 Free PMC article.
-
A single immunization with a rhabdovirus-based vector expressing severe acute respiratory syndrome coronavirus (SARS-CoV) S protein results in the production of high levels of SARS-CoV-neutralizing antibodies.J Gen Virol. 2005 May;86(Pt 5):1435-1440. doi: 10.1099/vir.0.80844-0. J Gen Virol. 2005. PMID: 15831955 Free PMC article.
Cited by
-
The critical impacts of cytokine storms in respiratory disorders.Heliyon. 2024 Apr 17;10(9):e29769. doi: 10.1016/j.heliyon.2024.e29769. eCollection 2024 May 15. Heliyon. 2024. PMID: 38694122 Free PMC article. Review.
-
Stockholm Syndrome: How to come to peace with our captor.J Assoc Med Microbiol Infect Dis Can. 2020 Dec 31;5(4):209-213. doi: 10.3138/jammi-2020-10-07. eCollection 2020 Dec. J Assoc Med Microbiol Infect Dis Can. 2020. PMID: 36340055 Free PMC article. No abstract available.
-
Vaccine Preventable Zoonotic Diseases: Challenges and Opportunities for Public Health Progress.Vaccines (Basel). 2022 Jun 22;10(7):993. doi: 10.3390/vaccines10070993. Vaccines (Basel). 2022. PMID: 35891157 Free PMC article. Review.
-
A Novel Prophylaxis Strategy Using Liposomal Vaccine Adjuvant CAF09b Protects against Influenza Virus Disease.Int J Mol Sci. 2022 Feb 6;23(3):1850. doi: 10.3390/ijms23031850. Int J Mol Sci. 2022. PMID: 35163772 Free PMC article.
-
Nanoparticle and virus-like particle vaccine approaches against SARS-CoV-2.J Microbiol. 2022 Mar;60(3):335-346. doi: 10.1007/s12275-022-1608-z. Epub 2022 Jan 28. J Microbiol. 2022. PMID: 35089583 Free PMC article. Review.
References
-
- Fowler R.A., Lapinsky S.E., Hallett D., Detsky A.S., Sibbald W.J., Slutsky A.S., Toronto SARS Critical Care Group Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290(3):367–373. - PubMed
-
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953–1966. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

