Distinct functional sites for human immunodeficiency virus type 1 and stromal cell-derived factor 1alpha on CXCR4 transmembrane helical domains
- PMID: 16188969
- PMCID: PMC1235829
- DOI: 10.1128/JVI.79.20.12667-12673.2005
Distinct functional sites for human immunodeficiency virus type 1 and stromal cell-derived factor 1alpha on CXCR4 transmembrane helical domains
Abstract
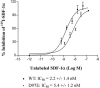
The entry of human immunodeficiency virus type 1 (HIV-1) into the cell is initiated by the interaction of the viral surface envelope protein with two cell surface components of the target cell, CD4 and a chemokine coreceptor, usually CXCR4 or CCR5. The natural ligand of CXCR4 is stromal cell-derived factor 1alpha (SDF-1alpha). Whereas the overlap between HIV-1 and SDF-1alpha functional sites on the extracellular domains of CXCR4 has been well documented, it has yet to be determined whether there are sites in the transmembrane (TM) helices of CXCR4 important for HIV-1 and/or SDF-1alpha functions, and if such sites do exist, whether they are overlapping or distinctive for the separate functions of CXCR4. For this study, by employing alanine-scanning mutagenesis, (125)I-SDF-1alpha competition binding, Ca(2+) mobilization, and cell-cell fusion assays, we found that the mutation of many CXCR4 TM residues, including Tyr(45), His(79), Asp(97), Pro(163), Trp(252), Tyr(255), Asp(262), Glu(288), His(294), and Asn(298), could selectively decrease HIV-1-mediated cell fusion but not the binding activity of SDF-1alpha. Phe(87) and Phe(292), which were involved in SDF-1alpha binding, did not play a significant role in the coreceptor activity of CXCR4, further demonstrating the disconnection between physiological and pathological activities of CXCR4 TM domains. Our data also show that four mutations of the second extracellular loop, D182A, D187A, F189A, and P191A, could reduce HIV-1 entry without impairing either ligand binding or signaling. Taken together, our first detailed characterization of the different functional roles of CXCR4 TM domains may suggest a mechanistic basis for the discovery of new selective anti-HIV agents.
Figures

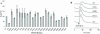
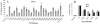
Similar articles
-
Unique ligand binding sites on CXCR4 probed by a chemical biology approach: implications for the design of selective human immunodeficiency virus type 1 inhibitors.J Virol. 2005 Dec;79(24):15398-404. doi: 10.1128/JVI.79.24.15398-15404.2005. J Virol. 2005. PMID: 16306611 Free PMC article.
-
Identification of residues of CXCR4 critical for human immunodeficiency virus coreceptor and chemokine receptor activities.J Biol Chem. 2000 Aug 4;275(31):23736-44. doi: 10.1074/jbc.M000776200. J Biol Chem. 2000. PMID: 10825158
-
Glycans and proteoglycans are involved in the interactions of human immunodeficiency virus type 1 envelope glycoprotein and of SDF-1alpha with membrane ligands of CD4(+) CXCR4(+) cells.Virology. 1999 Dec 20;265(2):354-64. doi: 10.1006/viro.1999.0033. Virology. 1999. PMID: 10600606
-
Synthetic peptides for study of human immunodeficiency virus infection.Appl Biochem Biotechnol. 2002 Jul-Dec;102-103(1-6):41-7. doi: 10.1385/abab:102-103:1-6:041. Appl Biochem Biotechnol. 2002. PMID: 12396109 Review.
-
Human immunodeficiency virus type 1 entry and chemokine receptors: a new therapeutic target.Antivir Chem Chemother. 1999 Mar;10(2):53-62. doi: 10.1177/095632029901000201. Antivir Chem Chemother. 1999. PMID: 10335399 Review.
Cited by
-
Research progress and applications of nanobody in human infectious diseases.Front Pharmacol. 2022 Aug 12;13:963978. doi: 10.3389/fphar.2022.963978. eCollection 2022. Front Pharmacol. 2022. PMID: 36034845 Free PMC article. Review.
-
Dual targeting of the chemokine receptors CXCR4 and ACKR3 with novel engineered chemokines.J Biol Chem. 2015 Sep 11;290(37):22385-97. doi: 10.1074/jbc.M115.675108. Epub 2015 Jul 27. J Biol Chem. 2015. PMID: 26216880 Free PMC article.
-
Functional anatomy of the full-length CXCR4-CXCL12 complex systematically dissected by quantitative model-guided mutagenesis.Sci Signal. 2020 Jul 14;13(640):eaay5024. doi: 10.1126/scisignal.aay5024. Sci Signal. 2020. PMID: 32665413 Free PMC article.
-
Biology and clinical relevance of chemokines and chemokine receptors CXCR4 and CCR5 in human diseases.Exp Biol Med (Maywood). 2011 Jun 1;236(6):637-47. doi: 10.1258/ebm.2011.010389. Epub 2011 May 12. Exp Biol Med (Maywood). 2011. PMID: 21565895 Free PMC article. Review.
-
Identifying and Assessing Putative Allosteric Sites and Modulators for CXCR4 Predicted through Network Modeling and Site Identification by Ligand Competitive Saturation.J Phys Chem B. 2024 May 30;128(21):5157-5174. doi: 10.1021/acs.jpcb.4c00925. Epub 2024 Apr 22. J Phys Chem B. 2024. PMID: 38647430 Free PMC article.
References
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
-
- Blanpain, C., B. J. Doranz, A. Bondue, C. Govaerts, A. De Leener, G. Vassart, R. W. Doms, A. Proudfoot, and M. Parmentier. 2003. The core domain of chemokines binds CCR5 extracellular domains while their amino terminus interacts with the transmembrane helix bundle. J. Biol. Chem. 278:5179-5187. - PubMed
-
- Bleul, C. C., M. Farzan, H. Choe, C. Parolin, I. Clark-Lewis, and J. Sodroski. 1996. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382:829-833. - PubMed
-
- Brelot, A., N. Heveker, M. Montes, and M. Alizon. 2000. Identification of residues of CXCR4 critical for human immunodeficiency virus coreceptor and chemokine receptor activities. J. Biol. Chem. 275:23736-23744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

