The vacuolar DHHC-CRD protein Pfa3p is a protein acyltransferase for Vac8p
- PMID: 16186255
- PMCID: PMC2171546
- DOI: 10.1083/jcb.200507048
The vacuolar DHHC-CRD protein Pfa3p is a protein acyltransferase for Vac8p
Abstract
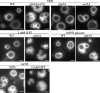
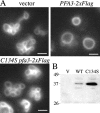
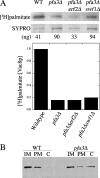
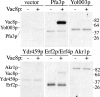
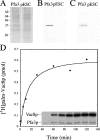
Palmitoylation of the vacuolar membrane protein Vac8p is essential for vacuole fusion in yeast (Veit, M., R. Laage, L. Dietrich, L. Wang, and C. Ungermann. 2001. EMBO J. 20:3145-3155; Wang, Y.X., E.J. Kauffman, J.E. Duex, and L.S. Weisman. 2001. J. Biol. Chem. 276:35133-35140). Proteins that contain an Asp-His-His-Cys (DHHC)-cysteine rich domain (CRD) are emerging as a family of protein acyltransferases, and are therefore candidates for mediators of Vac8p palmitoylation. Here we demonstrate that the DHHC-CRD proteins Pfa3p (protein fatty acyltransferase 3, encoded by YNL326c) and Swf1p are important for vacuole fusion. Cells lacking Pfa3p had fragmented vacuoles when stressed, and cells lacking both Pfa3p and Swf1p had fragmented vacuoles under normal growth conditions. Pfa3p promoted Vac8p membrane association and palmitoylation in vivo and partially purified Pfa3p palmitoylated Vac8p in vitro, establishing Vac8p as a substrate for palmitoylation by Pfa3p. Vac8p is the first N-myristoylated, palmitoylated protein identified as a substrate for a DHHC-CRD protein.
Figures


Similar articles
-
Vac8p release from the SNARE complex and its palmitoylation are coupled and essential for vacuole fusion.EMBO J. 2001 Jun 15;20(12):3145-55. doi: 10.1093/emboj/20.12.3145. EMBO J. 2001. PMID: 11406591 Free PMC article.
-
A novel cold-sensitive allele of the rate-limiting enzyme of fatty acid synthesis, acetyl coenzyme A carboxylase, affects the morphology of the yeast vacuole through acylation of Vac8p.Mol Cell Biol. 2000 May;20(9):2984-95. doi: 10.1128/MCB.20.9.2984-2995.2000. Mol Cell Biol. 2000. PMID: 10757783 Free PMC article.
-
Palmitoylation plays a role in targeting Vac8p to specific membrane subdomains.Traffic. 2006 Oct;7(10):1378-87. doi: 10.1111/j.1600-0854.2006.00472.x. Traffic. 2006. PMID: 16978392
-
Probing protein palmitoylation at the yeast vacuole.Methods. 2006 Oct;40(2):171-6. doi: 10.1016/j.ymeth.2006.06.020. Methods. 2006. PMID: 17012029 Review.
-
Protein lipidation.FEBS J. 2007 Oct;274(20):5202-10. doi: 10.1111/j.1742-4658.2007.06056.x. Epub 2007 Sep 24. FEBS J. 2007. PMID: 17892486 Review.
Cited by
-
Identification of conserved regions and residues within Hedgehog acyltransferase critical for palmitoylation of Sonic Hedgehog.PLoS One. 2010 Jun 23;5(6):e11195. doi: 10.1371/journal.pone.0011195. PLoS One. 2010. PMID: 20585641 Free PMC article.
-
The intracellular dynamic of protein palmitoylation.J Cell Biol. 2010 Dec 27;191(7):1229-38. doi: 10.1083/jcb.201008160. J Cell Biol. 2010. PMID: 21187327 Free PMC article. Review.
-
Yeast TLDc domain proteins regulate assembly state and subcellular localization of the V-ATPase.EMBO J. 2024 May;43(9):1870-1897. doi: 10.1038/s44318-024-00097-2. Epub 2024 Apr 8. EMBO J. 2024. PMID: 38589611 Free PMC article.
-
Protein palmitoylation: Palmitoyltransferases and their specificity.Exp Biol Med (Maywood). 2017 Jun;242(11):1150-1157. doi: 10.1177/1535370217707732. Epub 2017 May 9. Exp Biol Med (Maywood). 2017. PMID: 28485685 Free PMC article.
-
S-acylation-dependent association of the calcium sensor CBL2 with the vacuolar membrane is essential for proper abscisic acid responses.Cell Res. 2012 Jul;22(7):1155-68. doi: 10.1038/cr.2012.71. Epub 2012 May 1. Cell Res. 2012. PMID: 22547024 Free PMC article.
References
-
- Adams, A., D.E. Gottschling, C.A. Kaiser, and T. Stearns. 1997. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Plainview, NY. 177 pp.
-
- Chen, D.C., B.C. Yang, and T.T. Kuo. 1992. One-step transformation of yeast in stationary phase. Curr. Genet. 21:83–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

