Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo
- PMID: 16184195
- PMCID: PMC1224297
- DOI: 10.1172/JCI24970
Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo
Abstract
Disruption of the intestinal epithelial barrier occurs in many intestinal diseases, but neither the mechanisms nor the contribution of barrier dysfunction to disease pathogenesis have been defined. We utilized a murine model of T cell-mediated acute diarrhea to investigate the role of the epithelial barrier in diarrheal disease. We show that epithelial barrier dysfunction is required for the development of diarrhea. This diarrhea is characterized by reversal of net water flux, from absorption to secretion; increased leak of serum protein into the intestinal lumen; and altered tight junction structure. Phosphorylation of epithelial myosin II regulatory light chain (MLC), which has been correlated with tight junction regulation in vitro, increased abruptly after T cell activation and coincided with the development of diarrhea. Genetic knockout of long myosin light chain kinase (MLCK) or treatment of wild-type mice with a highly specific peptide MLCK inhibitor prevented epithelial MLC phosphorylation, tight junction disruption, protein leak, and diarrhea following T cell activation. These data show that epithelial MLCK is essential for intestinal barrier dysfunction and that this barrier dysfunction is critical to pathogenesis of diarrheal disease. The data also indicate that inhibition of epithelial MLCK may be an effective non-immunosuppressive therapy for treatment of immune-mediated intestinal disease.
Figures



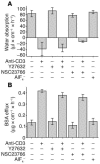



Similar articles
-
Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo.J Clin Invest. 2006 Oct;116(10):2682-94. doi: 10.1172/JCI29218. J Clin Invest. 2006. PMID: 17016558 Free PMC article.
-
IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction.Gastroenterology. 2006 Oct;131(4):1153-63. doi: 10.1053/j.gastro.2006.08.022. Epub 2006 Aug 22. Gastroenterology. 2006. PMID: 17030185 Free PMC article.
-
Myosin light chain kinase mediates intestinal barrier dysfunction via occludin endocytosis during anoxia/reoxygenation injury.Am J Physiol Cell Physiol. 2016 Dec 1;311(6):C996-C1004. doi: 10.1152/ajpcell.00113.2016. Epub 2016 Oct 19. Am J Physiol Cell Physiol. 2016. PMID: 27760753
-
Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application.Am J Pathol. 2006 Dec;169(6):1901-9. doi: 10.2353/ajpath.2006.060681. Am J Pathol. 2006. PMID: 17148655 Free PMC article. Review.
-
The Regulation of Intestinal Mucosal Barrier by Myosin Light Chain Kinase/Rho Kinases.Int J Mol Sci. 2020 May 18;21(10):3550. doi: 10.3390/ijms21103550. Int J Mol Sci. 2020. PMID: 32443411 Free PMC article. Review.
Cited by
-
Balancing Tumor Immunotherapy and Immune-Related Adverse Events: Unveiling the Key Regulators.Int J Mol Sci. 2024 Oct 10;25(20):10919. doi: 10.3390/ijms252010919. Int J Mol Sci. 2024. PMID: 39456702 Free PMC article. Review.
-
Challenges in IBD Research 2024: Preclinical Human IBD Mechanisms.Inflamm Bowel Dis. 2024 May 23;30(Supplement_2):S5-S18. doi: 10.1093/ibd/izae081. Inflamm Bowel Dis. 2024. PMID: 38778627 Free PMC article. Review.
-
Molecular mechanism of MLCK1 inducing 5-Fu resistance in colorectal cancer cells through activation of TNFR2/NF-κB pathway.Discov Oncol. 2024 May 12;15(1):159. doi: 10.1007/s12672-024-01019-8. Discov Oncol. 2024. PMID: 38735014 Free PMC article.
-
A short guide to the tight junction.J Cell Sci. 2024 May 1;137(9):jcs261776. doi: 10.1242/jcs.261776. Epub 2024 May 7. J Cell Sci. 2024. PMID: 38712627 Free PMC article. Review.
-
The barrier-protective effect of β-eudesmol against type 2-inflammatory cytokine-induced tight junction disassembly in airway epithelial cells.PLoS One. 2024 Apr 30;19(4):e0302851. doi: 10.1371/journal.pone.0302851. eCollection 2024. PLoS One. 2024. PMID: 38687777 Free PMC article.
References
-
- Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. - PubMed
-
- Brown GR, et al. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology. 1999;116:593–601. - PubMed
-
- Breslin NP, et al. Intestinal permeability is increased in a proportion of spouses of patients with Crohn’s disease. Am. J. Gastroenterol. 2001;96:2934–2938. - PubMed
-
- Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437–1439. - PubMed
-
- Yacyshyn BR, Meddings JB. CD45RO expression on circulating CD19+ B cells in Crohn’s disease correlates with intestinal permeability. Gastroenterology. 1995;108:132–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30DK42086/DK/NIDDK NIH HHS/United States
- P30 DK042086/DK/NIDDK NIH HHS/United States
- R01 DK054778/DK/NIDDK NIH HHS/United States
- R01 DK048816/DK/NIDDK NIH HHS/United States
- P30CA14599/CA/NCI NIH HHS/United States
- P30 CA014599/CA/NCI NIH HHS/United States
- R01DK68271/DK/NIDDK NIH HHS/United States
- T32 GM007281/GM/NIGMS NIH HHS/United States
- R01 DK061931/DK/NIDDK NIH HHS/United States
- R01DK54778/DK/NIDDK NIH HHS/United States
- R01 DK068271/DK/NIDDK NIH HHS/United States
- R01DK48816/DK/NIDDK NIH HHS/United States
- R01DK61931/DK/NIDDK NIH HHS/United States
- R01 DK054778-05/DK/NIDDK NIH HHS/United States
- T32 GM07281/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

