Beta-glucan enhances complement-mediated hematopoietic recovery after bone marrow injury
- PMID: 16179370
- PMCID: PMC1895628
- DOI: 10.1182/blood-2005-07-2705
Beta-glucan enhances complement-mediated hematopoietic recovery after bone marrow injury
Abstract
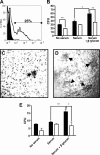
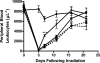
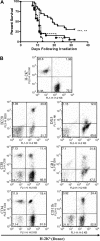
Myelotoxic injury in the bone marrow (BM) as a consequence of total body irradiation (TBI) or granulocyte colony-stimulating factor (G-CSF) mobilization results in the deposition of iC3b on BM stroma (stroma-iC3b). In the present study, we have examined how stroma-iC3b interacts with hematopoietic progenitor cells (HPCs) and the role of complement (C) and complement receptor 3 (CR3) in BM injury/repair. We demonstrate here that stroma-iC3b tethers HPCs via the inserted (I) domain of HPC complement receptor 3 (CR3, CD11b/CD18, Mac-1). Following irradiation, stroma-iC3b was observed in the presence of purified IgM and normal mouse serum (NMS), but not serum from Rag-2(-/-) mice, implicating a role for antibody (Ab) and the classic pathway of C activation. Furthermore, a novel role for soluble yeast beta-glucan, a ligand for the CR3 lectin-like domain (LLD), in the priming of CR3(+) HPC is suggested. Soluble yeast beta-glucan could enhance the proliferation of tethered HPCs, promote leukocyte recovery following sublethal irradiation, and increase the survival of lethally irradiated animals following allogeneic HPC transplantation in a CR3-dependent manner. Taken together, these observations suggest a novel role for C, CR3, and beta-glucan in the restoration of hematopoiesis following injury.
Figures

Similar articles
-
The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells.J Immunol. 1999 Feb 15;162(4):2281-90. J Immunol. 1999. PMID: 9973505
-
Beta-glucan, a "specific" biologic response modifier that uses antibodies to target tumors for cytotoxic recognition by leukocyte complement receptor type 3 (CD11b/CD18).J Immunol. 1999 Sep 15;163(6):3045-52. J Immunol. 1999. PMID: 10477568
-
Mobilization of hematopoietic progenitor cells by yeast-derived beta-glucan requires activation of matrix metalloproteinase-9.Stem Cells. 2008 May;26(5):1231-40. doi: 10.1634/stemcells.2007-0712. Epub 2008 Mar 13. Stem Cells. 2008. PMID: 18339771
-
Biologia Futura: stories about the functions of β2-integrins in human phagocytes.Biol Futur. 2021 Mar;72(1):7-13. doi: 10.1007/s42977-020-00063-z. Epub 2021 Feb 6. Biol Futur. 2021. PMID: 34554501 Review.
-
Contribution of CR3, CD11b/CD18 to cytolysis by human NK cells.Mol Immunol. 1990 Dec;27(12):1343-7. doi: 10.1016/0161-5890(90)90041-w. Mol Immunol. 1990. PMID: 1980339 Review.
Cited by
-
Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes.Leukemia. 2009 Nov;23(11):2052-62. doi: 10.1038/leu.2009.158. Epub 2009 Aug 6. Leukemia. 2009. PMID: 19657368 Free PMC article.
-
Maitake beta-glucan promotes recovery of leukocytes and myeloid cell function in peripheral blood from paclitaxel hematotoxicity.Cancer Immunol Immunother. 2010 Jun;59(6):885-97. doi: 10.1007/s00262-009-0815-3. Epub 2010 Feb 6. Cancer Immunol Immunother. 2010. PMID: 20140432 Free PMC article.
-
Combined yeast-derived beta-glucan with anti-tumor monoclonal antibody for cancer immunotherapy.Exp Mol Pathol. 2009 Jun;86(3):208-14. doi: 10.1016/j.yexmp.2009.01.006. Epub 2009 Jan 21. Exp Mol Pathol. 2009. PMID: 19454271 Free PMC article. Review.
-
Effects of glucan on bone marrow.Ann Transl Med. 2014 Feb;2(2):18. doi: 10.3978/j.issn.2305-5839.2014.01.06. Ann Transl Med. 2014. PMID: 25332994 Free PMC article. Review.
-
Maitake beta-glucan enhances umbilical cord blood stem cell transplantation in the NOD/SCID mouse.Exp Biol Med (Maywood). 2009 Mar;234(3):342-53. doi: 10.3181/0807-RM-226. Epub 2009 Jan 14. Exp Biol Med (Maywood). 2009. PMID: 19144872 Free PMC article.
References
-
- Carroll MC, Fischer MB. Complement and the immune response. Curr Opin Immunol. 1997;9: 64-69. - PubMed
-
- Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5: 981-986. - PubMed
-
- Ratajczak J, Reca R, Kucia M, et al. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103: 2071-2078. - PubMed
-
- Reca R, Mastellos D, Majka M, et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101: 3784-3793. - PubMed
-
- Ratajczak MZ, Reca R, Wysoczynski M, et al. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18: 1482-1490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

