Dynamic cycling of eIF2 through a large eIF2B-containing cytoplasmic body: implications for translation control
- PMID: 16157703
- PMCID: PMC2171431
- DOI: 10.1083/jcb.200503162
Dynamic cycling of eIF2 through a large eIF2B-containing cytoplasmic body: implications for translation control
Abstract
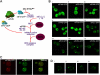
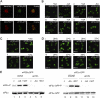
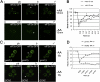
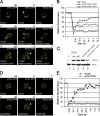
The eukaryotic translation initiation factor 2B (eIF2B) provides a fundamental controlled point in the pathway of protein synthesis. eIF2B is the heteropentameric guanine nucleotide exchange factor that converts eIF2, from an inactive guanosine diphosphate-bound complex to eIF2-guanosine triphosphate. This reaction is controlled in response to a variety of cellular stresses to allow the rapid reprogramming of cellular gene expression. Here we demonstrate that in contrast to other translation initiation factors, eIF2B and eIF2 colocalize to a specific cytoplasmic locus. The dynamic nature of this locus is revealed through fluorescence recovery after photobleaching analysis. Indeed eIF2 shuttles into these foci whereas eIF2B remains largely resident. Three different strategies to decrease the guanine nucleotide exchange function of eIF2B all inhibit eIF2 shuttling into the foci. These results implicate a defined cytoplasmic center of eIF2B in the exchange of guanine nucleotides on the eIF2 translation initiation factor. A focused core of eIF2B guanine nucleotide exchange might allow either greater activity or control of this elementary conserved step in the translation pathway.
Figures

Similar articles
-
The beta/Gcd7 subunit of eukaryotic translation initiation factor 2B (eIF2B), a guanine nucleotide exchange factor, is crucial for binding eIF2 in vivo.Mol Cell Biol. 2010 Nov;30(21):5218-33. doi: 10.1128/MCB.00265-10. Epub 2010 Aug 30. Mol Cell Biol. 2010. PMID: 20805354 Free PMC article.
-
Identification of domains and residues within the epsilon subunit of eukaryotic translation initiation factor 2B (eIF2Bepsilon) required for guanine nucleotide exchange reveals a novel activation function promoted by eIF2B complex formation.Mol Cell Biol. 2000 Jun;20(11):3965-76. doi: 10.1128/MCB.20.11.3965-3976.2000. Mol Cell Biol. 2000. PMID: 10805739 Free PMC article.
-
An eIF5/eIF2 complex antagonizes guanine nucleotide exchange by eIF2B during translation initiation.EMBO J. 2006 Oct 4;25(19):4537-46. doi: 10.1038/sj.emboj.7601339. Epub 2006 Sep 21. EMBO J. 2006. PMID: 16990799 Free PMC article.
-
Localization of the translational guanine nucleotide exchange factor eIF2B: a common theme for GEFs?Cell Cycle. 2006 Apr;5(7):678-80. doi: 10.4161/cc.5.7.2607. Epub 2006 Apr 1. Cell Cycle. 2006. PMID: 16582624 Review.
-
Protection of eIF2B from inhibitory phosphorylated eIF2: A viral strategy to maintain mRNA translation during the PKR-triggered integrated stress response.J Biol Chem. 2023 Nov;299(11):105287. doi: 10.1016/j.jbc.2023.105287. Epub 2023 Sep 22. J Biol Chem. 2023. PMID: 37742919 Free PMC article. Review.
Cited by
-
Control of Translation at the Initiation Phase During Glucose Starvation in Yeast.Int J Mol Sci. 2019 Aug 19;20(16):4043. doi: 10.3390/ijms20164043. Int J Mol Sci. 2019. PMID: 31430885 Free PMC article. Review.
-
Fusel alcohols regulate translation initiation by inhibiting eIF2B to reduce ternary complex in a mechanism that may involve altering the integrity and dynamics of the eIF2B body.Mol Biol Cell. 2010 Jul 1;21(13):2202-16. doi: 10.1091/mbc.e09-11-0962. Epub 2010 May 5. Mol Biol Cell. 2010. PMID: 20444979 Free PMC article.
-
RNA granules: the good, the bad and the ugly.Cell Signal. 2011 Feb;23(2):324-34. doi: 10.1016/j.cellsig.2010.08.011. Epub 2010 Sep 8. Cell Signal. 2011. PMID: 20813183 Free PMC article. Review.
-
Cellular stress leads to the formation of membraneless stress assemblies in eukaryotic cells.Traffic. 2019 Sep;20(9):623-638. doi: 10.1111/tra.12669. Epub 2019 Jul 30. Traffic. 2019. PMID: 31152627 Free PMC article. Review.
-
Relocalization of Translation Termination and Ribosome Recycling Factors to Stress Granules Coincides with Elevated Stop-Codon Readthrough and Reinitiation Rates upon Oxidative Stress.Cells. 2023 Jan 8;12(2):259. doi: 10.3390/cells12020259. Cells. 2023. PMID: 36672194 Free PMC article.
References
-
- Anderson, P., and N. Kedersha. 2002. Stressful Initiations. J. Cell Sci. 115:3227–3234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

