The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves
- PMID: 16155177
- PMCID: PMC1242264
- DOI: 10.1105/tpc.105.035766
The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves
Abstract
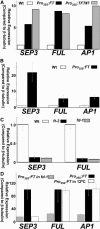
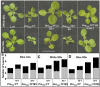
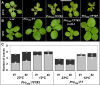
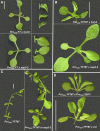
The transition to flowering involves major changes in the shoot apical meristem and in the fate of existing leaf primordia. Transcripts of the Arabidopsis thaliana flowering-promoting gene FLOWERING LOCUS T (FT) are present in leaf tissue but can also promote flowering when artificially introduced into the meristem. FT may normally act in the leaf and/or the meristem, initiating or constituting a mobile flower-promoting signal. We studied FT-dependent events in the rosette leaf, some of which might precede or mimic events in the meristem and its primordia. We show FT-dependent transcript accumulation of the MADS box transcription factors FRUITFULL (FUL) and SEPALLATA3 (SEP3) in leaves. Abnormally high levels of FT further increase the expression of these genes, leading to morphological changes in the leaves. Loss of the flowering-time gene FD, as well as environmental conditions that delay flowering, reduce FT's effect on leaves via reduced activation of its targets. FUL, SEP3, and APETALA1 accumulation in the meristem is associated with and contributes to the transition to flowering. We propose that FT functions through partner-dependent transcriptional activation of these and as-yet-unknown genes and that this occurs at several sites. Organ fate may depend on both degree of activation and the developmental stage reached by the organ before activation occurs.
Figures


Similar articles
-
FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex.Science. 2005 Aug 12;309(5737):1052-6. doi: 10.1126/science.1115983. Science. 2005. PMID: 16099979
-
Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis.Plant J. 2009 Nov;60(4):614-25. doi: 10.1111/j.1365-313X.2009.03986.x. Epub 2009 Jul 25. Plant J. 2009. PMID: 19656342
-
[Studying the role of FASCIATA5 gene in the regulation of flower development in Arabidopsis thaliana].Ontogenez. 2015 Jan-Feb;46(1):22-30. Ontogenez. 2015. PMID: 25898531 Russian.
-
The quest for florigen: a review of recent progress.J Exp Bot. 2006;57(13):3395-403. doi: 10.1093/jxb/erl095. Epub 2006 Oct 9. J Exp Bot. 2006. PMID: 17030536 Review.
-
Make hay when the sun shines: the role of MADS-box genes in temperature-dependant seasonal flowering responses.Plant Sci. 2011 Mar;180(3):447-53. doi: 10.1016/j.plantsci.2010.12.001. Epub 2010 Dec 14. Plant Sci. 2011. PMID: 21421391 Review.
Cited by
-
VEGETATIVE1 is essential for development of the compound inflorescence in pea.Nat Commun. 2012 Apr 24;3:797. doi: 10.1038/ncomms1801. Nat Commun. 2012. PMID: 22531182
-
Analysis of the Arabidopsis shoot meristem transcriptome during floral transition identifies distinct regulatory patterns and a leucine-rich repeat protein that promotes flowering.Plant Cell. 2012 Feb;24(2):444-62. doi: 10.1105/tpc.111.092791. Epub 2012 Feb 7. Plant Cell. 2012. PMID: 22319055 Free PMC article.
-
Novel roles for GIGANTEA revealed under environmental conditions that modify its expression in Arabidopsis and Medicago truncatula.Planta. 2006 Nov;224(6):1255-68. doi: 10.1007/s00425-006-0305-1. Epub 2006 Jun 15. Planta. 2006. PMID: 16775702
-
Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression.Plant Cell. 2011 Jun;23(6):2010-32. doi: 10.1105/tpc.111.084988. Epub 2011 Jun 3. Plant Cell. 2011. PMID: 21642548 Free PMC article. Review.
-
Sequential action of FRUITFULL as a modulator of the activity of the floral regulators SVP and SOC1.J Exp Bot. 2014 Mar;65(4):1193-203. doi: 10.1093/jxb/ert482. Epub 2014 Jan 24. J Exp Bot. 2014. PMID: 24465009 Free PMC article.
References
-
- Abe, M., Kobayashi, Y., Yamamoto, S., Daimon, Y., Yamaguchi, A., Ikeda, Y., Ichinoki, H., Notaguchi, M., Goto, K., and Araki, T. (2005). FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056. - PubMed
-
- An, H., Roussot, C., Suarez-Lopez, P., Corbesier, L., Vincent, C., Pineiro, M., Hepworth, S., Mouradov, A., Justin, S., Turnbull, C., and Coupland, G. (2004). CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131, 3615–3626. - PubMed
-
- Banfield, M.J., and Brady, R.L. (2000). The structure of Antirrhinum CENTRORADIALIS protein (CEN) suggests a role as a kinase regulator. J. Mol. Biol. 297, 1159–1170. - PubMed
-
- Blazquez, M.A., Ahn, J.H., and Weigel, D. (2003). A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat. Genet. 33, 168–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

