An activation domain within the walleye dermal sarcoma virus retroviral cyclin protein is essential for inhibition of the viral promoter
- PMID: 16150476
- PMCID: PMC3364292
- DOI: 10.1016/j.virol.2005.08.011
An activation domain within the walleye dermal sarcoma virus retroviral cyclin protein is essential for inhibition of the viral promoter
Abstract
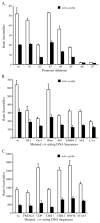
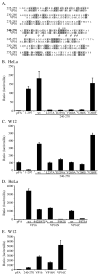
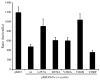
Walleye dermal sarcoma virus (WDSV) is a complex retrovirus associated with seasonal dermal sarcomas. Developing tumors have low levels of accessory gene transcripts, A1 and B, and regressing tumors have high levels of full-length and spliced transcripts. Transcript A1 encodes a retroviral cyclin (rv-cyclin) with limited homology to host cyclins. The rv-cyclin is physically linked to components of the transcriptional co-activator complex, Mediator, and regulates transcription. In walleye fibroblasts, it inhibits the WDSV promoter independently of cis-acting DNA sequences. The rv-cyclin activates transcription from GAL4 promoters when fused to the GAL4 DNA binding domain. A 30 a.a. activation domain in the carboxy region can be inactivated by single point mutations, and these mutations diminish the ability of the rv-cyclin to inhibit the WDSV promoter. When fused to glutathione S-transferase, the rv-cyclin, its carboxy region, and the activation domain pull down components of transcription complexes from nuclear extracts, and pull down is lost by mutation of the activation domain.
Figures



Similar articles
-
Retroviral cyclin controls cyclin-dependent kinase 8-mediated transcription elongation and reinitiation.J Virol. 2015 May;89(10):5450-61. doi: 10.1128/JVI.00464-15. Epub 2015 Mar 4. J Virol. 2015. PMID: 25741012 Free PMC article.
-
Walleye dermal sarcoma virus retroviral cyclin directly contacts TAF9.J Virol. 2006 Dec;80(24):12041-8. doi: 10.1128/JVI.01425-06. Epub 2006 Oct 11. J Virol. 2006. PMID: 17035330 Free PMC article.
-
Identification and characterization of cis-acting elements residing in the walleye dermal sarcoma virus promoter.J Virol. 2004 Jul;78(14):7590-601. doi: 10.1128/JVI.78.14.7590-7601.2004. J Virol. 2004. PMID: 15220434 Free PMC article.
-
Walleye dermal sarcoma virus cyclin interacts with components of the mediator complex and the RNA polymerase II holoenzyme.J Virol. 2002 Aug;76(16):8031-9. doi: 10.1128/jvi.76.16.8031-8039.2002. J Virol. 2002. PMID: 12134008 Free PMC article.
-
Comparative pathogenesis of epsilonretroviruses.J Virol. 2003 Dec;77(23):12385-91. doi: 10.1128/jvi.77.23.12385-12391.2003. J Virol. 2003. PMID: 14610162 Free PMC article. Review. No abstract available.
Cited by
-
Walleye dermal sarcoma virus: molecular biology and oncogenesis.Viruses. 2010 Sep;2(9):1984-1999. doi: 10.3390/v2091984. Epub 2010 Sep 22. Viruses. 2010. PMID: 21994717 Free PMC article.
-
Exploitation of the Mediator complex by viruses.PLoS Pathog. 2022 Apr 21;18(4):e1010422. doi: 10.1371/journal.ppat.1010422. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35446926 Free PMC article. No abstract available.
-
Retroviral cyclin enhances cyclin-dependent kinase-8 activity.J Virol. 2012 May;86(10):5742-51. doi: 10.1128/JVI.07006-11. Epub 2012 Feb 29. J Virol. 2012. PMID: 22379099 Free PMC article.
-
The retroviral cyclin of walleye dermal sarcoma virus binds cyclin-dependent kinases 3 and 8.Virology. 2011 Jan 20;409(2):299-307. doi: 10.1016/j.virol.2010.10.022. Epub 2010 Nov 9. Virology. 2011. PMID: 21067790 Free PMC article.
-
Retroviral cyclin controls cyclin-dependent kinase 8-mediated transcription elongation and reinitiation.J Virol. 2015 May;89(10):5450-61. doi: 10.1128/JVI.00464-15. Epub 2015 Mar 4. J Virol. 2015. PMID: 25741012 Free PMC article.
References
-
- Blazek E, Mittler G, Meisterernst M. The Mediator of RNA polymerase II. Chromosoma. 2005;113 (8):399–408. - PubMed
-
- Bowser PR, Wolfe MJ, Forney JL, Wooster GA. Seasonal prevalence of skin tumors from walleye (Stizostedion vitreum) from Oneida Lake, New York. J Wildl Dis. 1988;24:292–298. - PubMed
-
- Bowser PR, Wooster GA, Quackenbush SL, Casey RN, Casey JW. Comparison of fall and spring tumors as inocula for experimental transmission of walleye dermal sarcoma. J Aquat Anim Health. 1996;8:78–81.
-
- Breeden L, Nasmyth K. Regulation of the yeast HO gene. Cold Spring Harbor Symp Quant Biol. 1985;50:643–650. - PubMed
-
- Conaway JW, Florens L, Sato S, Tomomori-Sato C, Parmely TJ, Yao T, Swanson SK, Banks CA, Washburn MP, Conaway RC. The mammalian Mediator complex. FEBS Lett. 2005;579 (4):904–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

