Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines
- PMID: 16148231
- PMCID: PMC6725530
- DOI: 10.1523/JNEUROSCI.1808-05.2005
Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines
Abstract
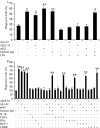
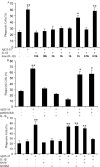
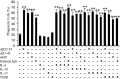
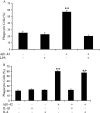
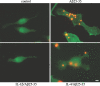
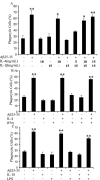
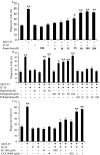
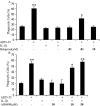
Microglia undergo a phenotypic activation in response to fibrillar beta-amyloid (fAbeta) deposition in the brains of Alzheimer's disease (AD) patients, resulting in their elaboration of inflammatory molecules. Despite the presence of abundant plaque-associated microglia in the brains of AD patients and in animal models of the disease, microglia fail to efficiently clear fAbeta deposits. However, they can be induced to do so during Abeta vaccination therapy attributable to anti-Abeta antibody stimulation of IgG receptor (FcR)-mediated phagocytic clearance of Abeta plaques. We report that proinflammatory cytokines attenuate microglial phagocytosis stimulated by fAbeta or complement receptor 3 and argue that this may, in part, underlie the accumulation of fAbeta-containing plaques within the AD brain. The proinflammatory suppression of fAbeta-elicited phagocytosis is dependent on nuclear factor kappaB activation. Significantly, the proinflammatory cytokines do not inhibit phagocytosis elicited by antibody-mediated activation of FcR, which may contribute to the efficiency of Abeta vaccination-based therapy. Importantly, the proinflammatory suppression of fAbeta phagocytosis can be relieved by the coincubation with anti-inflammatory cytokines, cyclooxygenase inhibitors, ibuprofen, or an E prostanoid receptor antagonist, suggesting that proinflammatory cytokines induce the production of prostaglandins, leading to an E prostanoid receptor-dependent inhibition of phagocytosis. These findings support anti-inflammatory therapies for the treatment of AD.
Figures
Similar articles
-
Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism.J Neurosci. 2004 Nov 3;24(44):9838-46. doi: 10.1523/JNEUROSCI.2557-04.2004. J Neurosci. 2004. PMID: 15525768 Free PMC article.
-
Complement component C3 and complement receptor type 3 contribute to the phagocytosis and clearance of fibrillar Aβ by microglia.Glia. 2012 May;60(6):993-1003. doi: 10.1002/glia.22331. Epub 2012 Mar 21. Glia. 2012. PMID: 22438044 Free PMC article.
-
Inhibition of STAT3- and MAPK-dependent PGE2 synthesis ameliorates phagocytosis of fibrillar β-amyloid peptide (1-42) via EP2 receptor in EMF-stimulated N9 microglial cells.J Neuroinflammation. 2016 Nov 21;13(1):296. doi: 10.1186/s12974-016-0762-9. J Neuroinflammation. 2016. PMID: 27871289 Free PMC article.
-
Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: implications for Alzheimer's disease.Mol Neurodegener. 2011 Jun 30;6:45. doi: 10.1186/1750-1326-6-45. Mol Neurodegener. 2011. PMID: 21718498 Free PMC article.
-
Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo.J Neurosci. 1998 Mar 15;18(6):2161-73. doi: 10.1523/JNEUROSCI.18-06-02161.1998. J Neurosci. 1998. PMID: 9482801 Free PMC article. Review.
Cited by
-
Increased tauopathy drives microglia-mediated clearance of beta-amyloid.Acta Neuropathol Commun. 2016 Jun 23;4(1):63. doi: 10.1186/s40478-016-0336-1. Acta Neuropathol Commun. 2016. PMID: 27339073 Free PMC article.
-
Research Progress on the Pathogenesis, Diagnosis, and Drug Therapy of Alzheimer's Disease.Brain Sci. 2024 Jun 9;14(6):590. doi: 10.3390/brainsci14060590. Brain Sci. 2024. PMID: 38928590 Free PMC article. Review.
-
Microglia subtypes show substrate- and time-dependent phagocytosis preferences and phenotype plasticity.Front Immunol. 2022 Aug 29;13:945485. doi: 10.3389/fimmu.2022.945485. eCollection 2022. Front Immunol. 2022. PMID: 36105813 Free PMC article.
-
Microglia in Aging and Alzheimer's Disease: A Comparative Species Review.Cells. 2021 May 8;10(5):1138. doi: 10.3390/cells10051138. Cells. 2021. PMID: 34066847 Free PMC article. Review.
-
Brain CB₂ Receptors: Implications for Neuropsychiatric Disorders.Pharmaceuticals (Basel). 2010 Aug 10;3(8):2517-2553. doi: 10.3390/ph3082517. Pharmaceuticals (Basel). 2010. PMID: 27713365 Free PMC article. Review.
References
-
- Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, Thomas RG, Weiner MF, Farlow MR, Sano M, Grundman M, Thal LJ (2000) A randomized controlled trial of prednisone in Alzheimer's disease. Alzheimer's Disease Cooperative Study. Neurology 54: 588–593. - PubMed
-
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ (2003) Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 289: 2819–2826. - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, et al. (2000) Inflammation and Alzheimer's disease. Neurobiol Aging 21: 383–421. - PMC - PubMed
-
- Arendash GW, Gordon MN, Diamond DM, Austin LA, Hatcher JM, Jantzen P, DiCarlo G, Wilcock D, Morgan D (2001) Behavioral assessment of Alzheimer's transgenic mice following long-term Abeta vaccination: task specificity and correlations between Abeta deposition and spatial memory. DNA Cell Biol 20: 737–744. - PubMed
-
- Aronoff DM, Canetti C, Peters-Golden M (2004) Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol 173: 559–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

