A novel glyco-conjugate vaccine against fungal pathogens
- PMID: 16147975
- PMCID: PMC2212864
- DOI: 10.1084/jem.20050749
A novel glyco-conjugate vaccine against fungal pathogens
Abstract
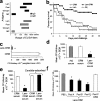
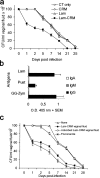
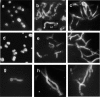
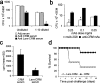
To generate a vaccine to protect against a variety of human pathogenic fungi, we conjugated laminarin (Lam), a well-characterized but poorly immunogenic beta-glucan preparation from the brown alga Laminaria digitata, with the diphtheria toxoid CRM197, a carrier protein used in some glyco-conjugate bacterial vaccines. This Lam-CRM conjugate proved to be immunogenic and protective as immunoprophylactic vaccine against both systemic and mucosal (vaginal) infections by Candida albicans. Protection probably was mediated by anti-beta-glucan antibodies as demonstrated by passive transfer of protection to naive mice by the whole immune serum, the immune vaginal fluid, and the affinity-purified anti-beta-glucan IgG fractions, as well as by administration of a beta-glucan-directed IgG2b mAb. Passive protection was prevented by adsorption of antibodies on Candida cells or beta-glucan particles before transfer. Anti-beta-glucan antibodies bound to C. albicans hyphae and inhibited their growth in vitro in the absence of immune-effector cells. Remarkably, Lam-CRM-vaccinated mice also were protected from a lethal challenge with conidia of Aspergillus fumigatus, and their serum also bound to and markedly inhibited the growth of A. fumigatus hyphae. Thus, this novel conjugate vaccine can efficiently immunize and protect against two major fungal pathogens by mechanisms that may include direct antifungal properties of anti-beta-glucan antibodies.
Figures


Similar articles
-
A beta-glucan-conjugate vaccine and anti-beta-glucan antibodies are effective against murine vaginal candidiasis as assessed by a novel in vivo imaging technique.Vaccine. 2010 Feb 17;28(7):1717-25. doi: 10.1016/j.vaccine.2009.12.021. Epub 2009 Dec 25. Vaccine. 2010. PMID: 20038431
-
Whole glucan particles as a vaccine against murine aspergillosis.J Med Microbiol. 2014 Dec;63(Pt 12):1750-1759. doi: 10.1099/jmm.0.079681-0. Epub 2014 Oct 6. J Med Microbiol. 2014. PMID: 25288643
-
Protection by anti-beta-glucan antibodies is associated with restricted beta-1,3 glucan binding specificity and inhibition of fungal growth and adherence.PLoS One. 2009;4(4):e5392. doi: 10.1371/journal.pone.0005392. Epub 2009 Apr 28. PLoS One. 2009. PMID: 19399183 Free PMC article.
-
Developing a vaccine against aspergillosis.Med Mycol. 2011 Apr;49 Suppl 1:S170-6. doi: 10.3109/13693786.2010.497775. Epub 2010 Jul 7. Med Mycol. 2011. PMID: 20608783 Review.
-
Potential of anti-Candida antibodies in immunoprophylaxis.Immunotherapy. 2010 Mar;2(2):171-83. doi: 10.2217/imt.09.76. Immunotherapy. 2010. PMID: 20635926 Review.
Cited by
-
Vaccine-Induced Immunological Memory in Invasive Fungal Infections - A Dream so Close yet so Far.Front Immunol. 2021 Apr 21;12:671068. doi: 10.3389/fimmu.2021.671068. eCollection 2021. Front Immunol. 2021. PMID: 33968079 Free PMC article. Review.
-
Vaccines for human fungal diseases: close but still a long way to go.NPJ Vaccines. 2021 Mar 3;6(1):33. doi: 10.1038/s41541-021-00294-8. NPJ Vaccines. 2021. PMID: 33658522 Free PMC article. Review.
-
Host defense pathways against fungi: the basis for vaccines and immunotherapy.Front Microbiol. 2012 May 10;3:176. doi: 10.3389/fmicb.2012.00176. eCollection 2012. Front Microbiol. 2012. PMID: 22590466 Free PMC article.
-
Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance.Chem Rev. 2022 Oct 26;122(20):15672-15716. doi: 10.1021/acs.chemrev.2c00021. Epub 2022 May 24. Chem Rev. 2022. PMID: 35608633 Free PMC article. Review.
-
C. albicans increases cell wall mannoprotein, but not mannan, in response to blood, serum and cultivation at physiological temperature.Glycobiology. 2011 Sep;21(9):1173-80. doi: 10.1093/glycob/cwr051. Epub 2011 Apr 21. Glycobiology. 2011. PMID: 21515585 Free PMC article.
References
-
- Edmond, M.B., S.E. Wallace, D.K. McClish, M.A. Pfaller, R.N. Jones, and R.P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three years analysis. Clin. Infect. Dis. 29:239–244. - PubMed
-
- Sanglard, D. 2002. Resistance of human fungal pathogens to antifungal drugs. Curr. Opin. Microbiol. 5:379–385. - PubMed
-
- Deepe, G.S. Jr. 2004. Preventative and therapeutic vaccines for fungal infections: from concept to implementation. Expert Rev. Vaccines. 3:1–9. - PubMed
-
- Mochon, A.B., and J.E. Cutler. 2005. Is a vaccine needed against Candida albicans? Med. Mycol. 43:97–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

