A novel role for GADD45beta as a mediator of MMP-13 gene expression during chondrocyte terminal differentiation
- PMID: 16144844
- PMCID: PMC3937966
- DOI: 10.1074/jbc.M504202200
A novel role for GADD45beta as a mediator of MMP-13 gene expression during chondrocyte terminal differentiation
Abstract
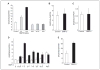
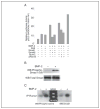
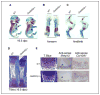
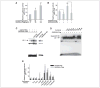
The growth arrest and DNA damage-inducible 45beta (GADD45beta) gene product has been implicated in the stress response, cell cycle arrest, and apoptosis. Here we demonstrated the unexpected expression of GADD45beta in the embryonic growth plate and uncovered its novel role as an essential mediator of matrix metalloproteinase-13 (MMP-13) expression during terminal chondrocyte differentiation. We identified GADD45beta as a prominent early response gene induced by bone morphogenetic protein-2 (BMP-2) through a Smad1/Runx2-dependent pathway. Because this pathway is involved in skeletal development, we examined mouse embryonic growth plates, and we observed expression of Gadd45beta mRNA coincident with Runx2 protein in pre-hypertrophic chondrocytes, whereas GADD45beta protein was localized prominently in the nucleus in late stage hypertrophic chondrocytes where Mmp-13 mRNA was expressed. In Gadd45beta(-/-) mouse embryos, defective mineralization and decreased bone growth accompanied deficient Mmp-13 and Col10a1 gene expression in the hypertrophic zone. Transduction of small interfering RNA-GADD45beta in epiphyseal chondrocytes in vitro blocked terminal differentiation and the associated expression of Mmp-13 and Col10a1 mRNA in vitro. Finally, GADD45beta stimulated MMP-13 promoter activity in chondrocytes through the JNK-mediated phosphorylation of JunD, partnered with Fra2, in synergy with Runx2. These observations indicated that GADD45beta plays an essential role during chondrocyte terminal differentiation.
Figures




Similar articles
-
Runx1/AML1/Cbfa2 mediates onset of mesenchymal cell differentiation toward chondrogenesis.J Bone Miner Res. 2005 Sep;20(9):1624-36. doi: 10.1359/JBMR.050516. Epub 2005 May 23. J Bone Miner Res. 2005. PMID: 16059634
-
Murine and chicken chondrocytes regulate osteoclastogenesis by producing RANKL in response to BMP2.J Bone Miner Res. 2008 Mar;23(3):314-25. doi: 10.1359/jbmr.071025. J Bone Miner Res. 2008. PMID: 17967138 Free PMC article.
-
Menin is required for bone morphogenetic protein 2- and transforming growth factor beta-regulated osteoblastic differentiation through interaction with Smads and Runx2.J Biol Chem. 2004 Sep 24;279(39):40267-75. doi: 10.1074/jbc.M401312200. Epub 2004 May 18. J Biol Chem. 2004. PMID: 15150273
-
Smad-Runx interactions during chondrocyte maturation.J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S15-22. J Bone Joint Surg Am. 2001. PMID: 11263661 Review.
-
Post-translational Regulation of Runx2 in Bone and Cartilage.J Dent Res. 2009 Aug;88(8):693-703. doi: 10.1177/0022034509341629. J Dent Res. 2009. PMID: 19734454 Free PMC article. Review.
Cited by
-
The increase in O-linked N-acetylglucosamine protein modification stimulates chondrogenic differentiation both in vitro and in vivo.J Biol Chem. 2012 Sep 28;287(40):33615-28. doi: 10.1074/jbc.M112.354241. Epub 2012 Aug 2. J Biol Chem. 2012. PMID: 22859309 Free PMC article.
-
GADD45 in Stress Signaling, Cell Cycle Control, and Apoptosis.Adv Exp Med Biol. 2022;1360:1-22. doi: 10.1007/978-3-030-94804-7_1. Adv Exp Med Biol. 2022. PMID: 35505159
-
Muscle cells enhance resistance to pro-inflammatory cytokine-induced cartilage destruction.Biochem Biophys Res Commun. 2010 Jan 29;392(1):22-8. doi: 10.1016/j.bbrc.2009.12.138. Epub 2009 Dec 31. Biochem Biophys Res Commun. 2010. PMID: 20043873 Free PMC article.
-
Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis.Eur Cell Mater. 2011 Feb 24;21:202-20. doi: 10.22203/ecm.v021a16. Eur Cell Mater. 2011. PMID: 21351054 Free PMC article.
-
Gadd45β expression in chondrosarcoma: a pilot study for diagnostic and biological implications in histological grading.Diagn Pathol. 2010 Oct 13;5:69. doi: 10.1186/1746-1596-5-69. Diagn Pathol. 2010. PMID: 20942912 Free PMC article.
References
-
- Abdollahi A, Lord KA, Hoffman-Liebermann B, Liebermann DA. Oncogene. 1991;6:165–167. - PubMed
-
- Amanullah A, Azam N, Balliet A, Hollander C, Hoffman B, Fornace A, Liebermann D. Nature. 2003;424:741–742. - PubMed
-
- Yoo J, Ghiassi M, Jirmanova L, Balliet AG, Hoffman B, Fornace AJ, Jr, Liebermann DA, Bottinger EP, Roberts AB. J Biol Chem. 2003;278:43001–43007. - PubMed
-
- Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L. Arthritis Rheum. 2001;44:2777–2789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

