Early block to erythromegakaryocytic development conferred by loss of transcription factor GATA-1
- PMID: 16144799
- PMCID: PMC1895362
- DOI: 10.1182/blood-2005-07-2740
Early block to erythromegakaryocytic development conferred by loss of transcription factor GATA-1
Abstract
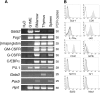
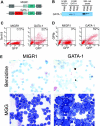
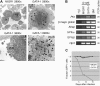
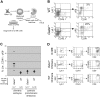
Transcription factor GATA-1 is essential at multiple stages of hematopoiesis. Murine gene targeting and analysis of naturally occurring human mutations demonstrate that GATA-1 drives the maturation of committed erythroid precursors and megakaryocytes. Prior studies also suggest additional, poorly defined, roles for GATA-1 at earlier stages of erythromegakaryocytic differentiation. To investigate these functions further, we stimulated Gata1- murine embryonic stem-cell-derived hematopoietic cultures with thrombopoietin, a multistage cytokine. Initially, the cultures generated a wave of mutant megakaryocytes. However, these were rapidly overgrown by a unique population of thrombopoietin-dependent blasts that express immature markers and proliferate indefinitely. Importantly, on restoration of GATA-1 function, these cells differentiated into both erythroid and megakaryocytic lineages, suggesting that they represent bipotential progenitors. Identical cells are also present in vivo, as indicated by flow cytometry and culture analysis of fetal livers from Gata1- chimeric mice. Our findings indicate that loss of GATA-1 impairs the maturation of megakaryocyte-erythroid progenitors. This defines a new role for GATA-1 at a relatively early stage of hematopoiesis and provides potential insight into recent discoveries that human GATA1 mutations promote acute megakaryoblastic leukemia, a clonal malignancy with features of both erythroid and megakaryocyte maturation.
Figures

Similar articles
-
Differential requirements for the activation domain and FOG-interaction surface of GATA-1 in megakaryocyte gene expression and development.Blood. 2005 Aug 15;106(4):1223-31. doi: 10.1182/blood-2005-02-0551. Epub 2005 Apr 28. Blood. 2005. PMID: 15860665 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
Ikaros inhibits megakaryopoiesis through functional interaction with GATA-1 and NOTCH signaling.Blood. 2013 Mar 28;121(13):2440-51. doi: 10.1182/blood-2012-08-450627. Epub 2013 Jan 18. Blood. 2013. PMID: 23335373 Free PMC article.
-
Cofactor-mediated restriction of GATA-1 chromatin occupancy coordinates lineage-specific gene expression.Mol Cell. 2012 Aug 24;47(4):608-21. doi: 10.1016/j.molcel.2012.05.051. Epub 2012 Jul 5. Mol Cell. 2012. PMID: 22771118 Free PMC article.
-
Polycomb repressive complex 2 (PRC2) restricts hematopoietic stem cell activity.PLoS Biol. 2008 Apr 15;6(4):e93. doi: 10.1371/journal.pbio.0060093. PLoS Biol. 2008. PMID: 18416604 Free PMC article.
-
Differential contribution of the Gata1 gene hematopoietic enhancer to erythroid differentiation.Mol Cell Biol. 2009 Mar;29(5):1163-75. doi: 10.1128/MCB.01572-08. Epub 2008 Dec 22. Mol Cell Biol. 2009. PMID: 19103751 Free PMC article.
-
Linkage between the mechanisms of thrombocytopenia and thrombopoiesis.Blood. 2016 Mar 10;127(10):1234-41. doi: 10.1182/blood-2015-07-607903. Epub 2016 Jan 19. Blood. 2016. PMID: 26787737 Free PMC article. Review.
References
-
- Weiss MJ, Orkin SH. GATA transcription factors: key regulators of hematopoiesis. Exp Hematol. 1995;23: 99-107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

