Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice
- PMID: 16140750
- PMCID: PMC1212608
- DOI: 10.1128/JVI.79.18.11724-11733.2005
Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice
Abstract
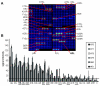
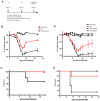
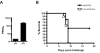
The smallpox vaccine is the prototypic vaccine, yet the viral targets critical for vaccine-mediated protection remain unclear in humans. We have produced protein microarrays of a near-complete vaccinia proteome and used them to determine the major antigen specificities of the human humoral immune response to the smallpox vaccine (Dryvax). H3L, an intracellular mature virion envelope protein, was consistently recognized by high-titer antibodies in the majority of human donors, particularly after secondary immunization. We then focused on examining H3L as a valuable human antibody target. Purified human anti-H3L antibodies exhibited substantial vaccinia virus-neutralizing activity in vitro (50% plaque reduction neutralization test [PRNT50] = 44 microg/ml). Mice also make an immunodominant antibody response to H3L after vaccination with vaccinia virus, as determined by vaccinia virus protein microarray. Mice were immunized with recombinant H3L protein to examine H3L-specific antibody responses in greater detail. H3L-immunized mice developed high-titer vaccinia virus-neutralizing antibodies (mean PRNT50 = 1:3,760). Importantly, H3L-immunized mice were subsequently protected against lethal intranasal challenges with 1 or 5 50% lethal doses (LD50) of pathogenic vaccinia virus strain WR, demonstrating the in vivo value of an anti-H3L response. To formally demonstrate that neutralizing anti-H3L antibodies are protective in vivo, we performed anti-H3L serum passive-transfer experiments. Mice receiving H3L-neutralizing antiserum were protected from a lethal challenge with 3 LD50 of vaccinia virus strain WR (5/10 versus 0/10; P < 0.02). Together, these data show that H3L is a major target of the human anti-poxvirus antibody response and is likely to be a key contributor to protection against poxvirus infection and disease.
Figures

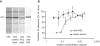

Similar articles
-
Immunogenicity and protective efficacy of recombinant major envelope protein (rH3L) of buffalopox virus in animal models.Antiviral Res. 2016 Feb;126:108-16. doi: 10.1016/j.antiviral.2015.12.004. Epub 2015 Dec 23. Antiviral Res. 2016. PMID: 26723250
-
Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge.J Virol. 2008 Apr;82(7):3517-29. doi: 10.1128/JVI.01854-07. Epub 2008 Jan 16. J Virol. 2008. PMID: 18199639 Free PMC article.
-
Antibodies to the A27 protein of vaccinia virus neutralize and protect against infection but represent a minor component of Dryvax vaccine--induced immunity.J Infect Dis. 2007 Oct 1;196(7):1026-32. doi: 10.1086/520936. Epub 2007 Aug 20. J Infect Dis. 2007. PMID: 17763325
-
Antibody Recognition of Immunodominant Vaccinia Virus Envelope Proteins.Subcell Biochem. 2017;83:103-126. doi: 10.1007/978-3-319-46503-6_4. Subcell Biochem. 2017. PMID: 28271474 Review.
-
Monitoring of human immunological responses to vaccinia virus.Methods Mol Biol. 2004;269:243-66. doi: 10.1385/1-59259-789-0:243. Methods Mol Biol. 2004. PMID: 15114020 Review.
Cited by
-
Utilizing poxviral vectored vaccines for antibody induction-progress and prospects.Vaccine. 2013 Sep 6;31(39):4223-30. doi: 10.1016/j.vaccine.2013.05.091. Epub 2013 Jun 5. Vaccine. 2013. PMID: 23746455 Free PMC article. Review.
-
Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections.Cell. 2016 Oct 20;167(3):684-694.e9. doi: 10.1016/j.cell.2016.09.049. Cell. 2016. PMID: 27768891 Free PMC article.
-
CD8 T cells are essential for recovery from a respiratory vaccinia virus infection.J Immunol. 2012 Sep 1;189(5):2432-40. doi: 10.4049/jimmunol.1200799. Epub 2012 Jul 23. J Immunol. 2012. PMID: 22826318 Free PMC article.
-
A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost.Vaccine. 2007 Jan 26;25(7):1214-24. doi: 10.1016/j.vaccine.2006.10.009. Epub 2006 Oct 17. Vaccine. 2007. PMID: 17098336 Free PMC article.
-
Transcriptionally active PCR for antigen identification and vaccine development: in vitro genome-wide screening and in vivo immunogenicity.Mol Biochem Parasitol. 2008 Mar;158(1):32-45. doi: 10.1016/j.molbiopara.2007.11.009. Epub 2007 Nov 22. Mol Biochem Parasitol. 2008. PMID: 18164079 Free PMC article.
References
-
- Alcami, A., and G. L. Smith. 1992. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell 71:153-167. - PubMed
-
- Bachmann, M. F., U. Kalinke, A. Althage, G. Freer, C. Burkhart, H. Roost, M. Aguet, H. Hengartner, and R. M. Zinkernagel. 1997. The role of antibody concentration and avidity in antiviral protection. Science 276:2024-2027. - PubMed
-
- Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425-431. - PubMed
-
- Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100:9458-9463. - PMC - PubMed
-
- Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199-2202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

