Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors
- PMID: 16116450
- PMCID: PMC1380263
- DOI: 10.1038/nn1522
Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors
Abstract
In the postnatal subventricular zone (SVZ), local cues or signaling molecules released from neuroblasts limit the proliferation of glial fibrillary acidic protein (GFAP)-expressing progenitors thought to be stem cells. However, signals between SVZ cells have not been identified. We show that depolarization of neuroblasts induces nonsynaptic SNARE-independent GABA(A) receptor currents in GFAP-expressing cells, the time course of which depends on GABA uptake in acute mouse slices. We found that GABA(A) receptors are tonically activated in GFAP-expressing cells, consistent with the presence of spontaneous depolarizations in neuroblasts that are sufficient to induce GABA release. These data demonstrate the existence of nonsynaptic GABAergic signaling between neuroblasts and GFAP-expressing cells. Furthermore, we show that GABA(A) receptor activation in GFAP-expressing cells limits their progression through the cell cycle. Thus, as GFAP-expressing cells generate neuroblasts, GABA released from neuroblasts provides a feedback mechanism to control the proliferation of GFAP-expressing progenitors by activating GABA(A) receptors.
Figures
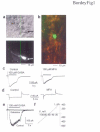
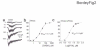


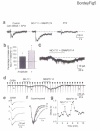
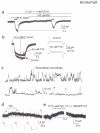
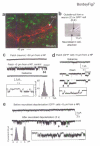
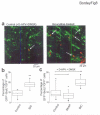
Comment in
-
GABA puts the brake on stem cells.Nat Neurosci. 2005 Sep;8(9):1132-3. doi: 10.1038/nn0905-1132. Nat Neurosci. 2005. PMID: 16127444 No abstract available.
Similar articles
-
Intraglomerular inhibition: signaling mechanisms of an olfactory microcircuit.Nat Neurosci. 2005 Mar;8(3):354-64. doi: 10.1038/nn1403. Epub 2005 Feb 6. Nat Neurosci. 2005. PMID: 15696160
-
GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes.Glia. 2006 Oct;54(5):394-410. doi: 10.1002/glia.20392. Glia. 2006. PMID: 16886203
-
GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain.Nat Neurosci. 2004 Nov;7(11):1233-41. doi: 10.1038/nn1340. Epub 2004 Oct 24. Nat Neurosci. 2004. PMID: 15494728
-
GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells.Neuron. 2005 Sep 15;47(6):803-15. doi: 10.1016/j.neuron.2005.08.023. Neuron. 2005. PMID: 16157276
-
Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain.J Physiol. 2008 Aug 15;586(16):3739-43. doi: 10.1113/jphysiol.2008.155325. Epub 2008 May 8. J Physiol. 2008. PMID: 18467361 Free PMC article. Review.
Cited by
-
GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury.Nat Neurosci. 2015 May;18(5):674-82. doi: 10.1038/nn.3990. Epub 2015 Mar 30. Nat Neurosci. 2015. PMID: 25821912 Free PMC article.
-
Environmental regulation of the chloride transporter KCC2: switching inflammation off to switch the GABA on?Transl Psychiatry. 2020 Oct 15;10(1):349. doi: 10.1038/s41398-020-01027-6. Transl Psychiatry. 2020. PMID: 33060559 Free PMC article. Review.
-
Partial inactivation of GABAA receptors containing the α5 subunit affects the development of adult-born dentate gyrus granule cells.Eur J Neurosci. 2016 Sep;44(5):2258-71. doi: 10.1111/ejn.13329. Epub 2016 Jul 19. Eur J Neurosci. 2016. PMID: 27364953 Free PMC article.
-
Lessons from the embryonic neural stem cell niche for neural lineage differentiation of pluripotent stem cells.Stem Cell Rev Rep. 2012 Sep;8(3):813-29. doi: 10.1007/s12015-012-9381-8. Stem Cell Rev Rep. 2012. PMID: 22628111 Free PMC article.
-
GABA Not Only a Neurotransmitter: Osmotic Regulation by GABA(A)R Signaling.Front Cell Neurosci. 2012 Jan 30;6:3. doi: 10.3389/fncel.2012.00003. eCollection 2011 Jan. Front Cell Neurosci. 2012. PMID: 22319472 Free PMC article.
References
-
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. - PubMed
-
- Luskin MB, Zigova T, Soteres BJ, Stewart RR. Neuronal progenitor cells derived from the anterior subventricular zone of the neonatal rat forebrain continue to proliferate in vitro and express a neuronal phenotype. Mol. Cell Neurosci. 1997;8:351–366. - PubMed
-
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 2003;6:507–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

