Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque
- PMID: 16116432
- PMCID: PMC7095788
- DOI: 10.1038/nm1280
Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque
Abstract
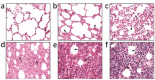
Development of therapeutic agents for severe acute respiratory syndrome (SARS) viral infection using short interfering RNA (siRNA) inhibitors exemplifies a powerful new means to combat emerging infectious diseases. Potent siRNA inhibitors of SARS coronavirus (SCV) in vitro were further evaluated for efficacy and safety in a rhesus macaque (Macaca mulatta) SARS model using clinically viable delivery while comparing three dosing regimens. Observations of SARS-like symptoms, measurements of SCV RNA presence and lung histopathology and immunohistochemistry consistently showed siRNA-mediated anti-SARS efficacy by either prophylactic or therapeutic regimens. The siRNAs used provided relief from SCV infection-induced fever, diminished SCV viral levels and reduced acute diffuse alveoli damage. The 10-40 mg/kg accumulated dosages of siRNA did not show any sign of siRNA-induced toxicity. These results suggest that a clinical investigation is warranted and illustrate the prospects for siRNA to enable a massive reduction in development time for new targeted therapeutic agents.
Conflict of interest statement
Bao-jian Li and Frank Y Xie are consultants for and Du Cheng is an employee of Guangzhou Top Genomics, Ltd. Qingquan Tang, Frank Y. Xie, Yijia Liu, Martin C. Woodle and Patrick Y. Liu are employees of Intradigm Corporation. Both Top Genomics, Ltd. and Intradigm Corporation are biopharmaceutical companies that are developing RNAi therapeutics for the treatment of human disease.
Figures

Similar articles
-
Application of siRNA against SARS in the rhesus macaque model.Methods Mol Biol. 2008;442:139-58. doi: 10.1007/978-1-59745-191-8_11. Methods Mol Biol. 2008. PMID: 18369784 Free PMC article.
-
Prophylactic and therapeutic effects of small interfering RNA targeting SARS-coronavirus.Antivir Ther. 2004 Jun;9(3):365-74. Antivir Ther. 2004. PMID: 15259899
-
Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques.Nat Med. 2004 Mar;10(3):290-3. doi: 10.1038/nm1001. Epub 2004 Feb 22. Nat Med. 2004. PMID: 14981511 Free PMC article.
-
Recent patents on treatment of severe acute respiratory syndrome (SARS).Recent Pat Antiinfect Drug Discov. 2007 Jan;2(1):1-10. doi: 10.2174/157489107779561698. Recent Pat Antiinfect Drug Discov. 2007. PMID: 18221160 Review.
-
Therapeutic and prophylactic potential of small interfering RNAs against severe acute respiratory syndrome: progress to date.BioDrugs. 2007;21(1):9-15. doi: 10.2165/00063030-200721010-00002. BioDrugs. 2007. PMID: 17263585 Free PMC article. Review.
Cited by
-
RNAi-based drug design: considerations and future directions.Nat Rev Drug Discov. 2024 May;23(5):341-364. doi: 10.1038/s41573-024-00912-9. Epub 2024 Apr 3. Nat Rev Drug Discov. 2024. PMID: 38570694 Free PMC article. Review.
-
A novel recombinant ORF7-siRNA delivered by flexible nano-liposomes inhibits varicella zoster virus infection.Cell Biosci. 2023 Sep 12;13(1):167. doi: 10.1186/s13578-023-01108-1. Cell Biosci. 2023. PMID: 37700336 Free PMC article.
-
Where should siRNAs go: applicable organs for siRNA drugs.Exp Mol Med. 2023 Jul;55(7):1283-1292. doi: 10.1038/s12276-023-00998-y. Epub 2023 Jul 10. Exp Mol Med. 2023. PMID: 37430086 Free PMC article. Review.
-
Innate immune regulations and various siRNA modalities.Drug Deliv Transl Res. 2023 Nov;13(11):2704-2718. doi: 10.1007/s13346-023-01361-4. Epub 2023 May 23. Drug Deliv Transl Res. 2023. PMID: 37219704 Free PMC article. Review.
-
Progress in non-viral localized delivery of siRNA therapeutics for pulmonary diseases.Acta Pharm Sin B. 2023 Apr;13(4):1400-1428. doi: 10.1016/j.apsb.2022.07.010. Epub 2022 Jul 19. Acta Pharm Sin B. 2023. PMID: 37139423 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
