G1 checkpoint failure and increased tumor susceptibility in mice lacking the novel p53 target Ptprv
- PMID: 16107883
- PMCID: PMC1201350
- DOI: 10.1038/sj.emboj.7600769
G1 checkpoint failure and increased tumor susceptibility in mice lacking the novel p53 target Ptprv
Abstract
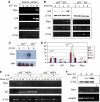
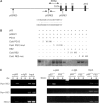
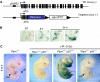
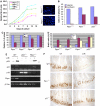
In response to DNA damage, p53 activates a G1 cell cycle checkpoint, in part through induction of the cyclin-dependent kinase inhibitor p21(Waf1/Cip1). Here we report the identification of a new direct p53 target, Ptprv (or ESP), encoding a transmembrane tyrosine phosphatase. Ptprv transcription is dramatically and preferentially increased in cultured cells undergoing p53-dependent cell cycle arrest, but not in cells undergoing p53-mediated apoptosis. This observation was further confirmed in vivo using a Ptprv null-reporter mouse line. A p53-responsive element is present in the Ptprv promoter and p53 is recruited to this site in vivo. Importantly, while p53-dependent apoptosis is intact in mice lacking Ptprv, Ptprv-null fibroblasts and epithelial cells of the small intestine are defective in G1 checkpoint control. Thus, Ptprv is a new direct p53 target and a key mediator of p53-induced cell cycle arrest. Finally, Ptprv loss enhances the formation of epidermal papillomas after exposure to chemical carcinogens, suggesting that Ptprv acts to suppress tumor formation in vivo.
Figures


Similar articles
-
PTPRV is a key mediator of p53-induced cell cycle exit.Cell Cycle. 2005 Dec;4(12):1703-5. doi: 10.4161/cc.4.12.2207. Epub 2005 Dec 26. Cell Cycle. 2005. PMID: 16258284
-
Selective loss of endogenous p21waf1/cip1 induction underlies the G1 checkpoint defect of monomeric p53 proteins.Oncogene. 1996 Aug 1;13(3):589-98. Oncogene. 1996. PMID: 8760300
-
WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis.Cancer Res. 1994 Mar 1;54(5):1169-74. Cancer Res. 1994. PMID: 8118801
-
[Cell cycle regulation after exposure to ionizing radiation].Bull Cancer. 1999 Apr;86(4):345-57. Bull Cancer. 1999. PMID: 10341340 Review. French.
-
The p53 tumour suppressor gene: a mediator of a G1 growth arrest and of apoptosis.Experientia. 1996 Oct 31;52(10-11):1001-7. doi: 10.1007/BF01920109. Experientia. 1996. PMID: 8917731 Review.
Cited by
-
Tumor-specific signaling to p53 is mimicked by Mdm2 inactivation in zebrafish: insights from mdm2 and mdm4 mutant zebrafish.Oncogene. 2015 Nov 26;34(48):5933-41. doi: 10.1038/onc.2015.57. Epub 2015 Mar 9. Oncogene. 2015. PMID: 25746004 Free PMC article.
-
Defective neurogenesis and schizophrenia-like behavior in PARP-1-deficient mice.Cell Death Dis. 2019 Dec 9;10(12):943. doi: 10.1038/s41419-019-2174-0. Cell Death Dis. 2019. PMID: 31819047 Free PMC article.
-
Unravelling mechanisms of p53-mediated tumour suppression.Nat Rev Cancer. 2014 May;14(5):359-70. doi: 10.1038/nrc3711. Epub 2014 Apr 17. Nat Rev Cancer. 2014. PMID: 24739573 Free PMC article.
-
Deconstructing networks of p53-mediated tumor suppression in vivo.Cell Death Differ. 2018 Jan;25(1):93-103. doi: 10.1038/cdd.2017.171. Epub 2017 Nov 3. Cell Death Differ. 2018. PMID: 29099489 Free PMC article. Review.
-
Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling.J Cell Biol. 2006 Mar 13;172(6):909-21. doi: 10.1083/jcb.200508130. J Cell Biol. 2006. PMID: 16533949 Free PMC article.
References
-
- Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T (2004) Protein tyrosine phosphatases in the human genome. Cell 117: 699–711 - PubMed
-
- Ben-Ze'ev A, Shtutman M, Zhurinsky J (2000) The integration of cell adhesion with gene expression: the role of beta-catenin. Exp Cell Res 261: 75–82 - PubMed
-
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ (1995) Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377: 552–557 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

