The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is required for cell entry and cell-cell fusion
- PMID: 16103150
- PMCID: PMC1193616
- DOI: 10.1128/JVI.79.17.10988-10998.2005
The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is required for cell entry and cell-cell fusion
Abstract
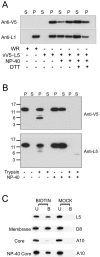
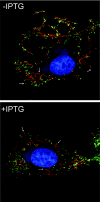
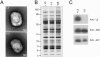
The L5R gene of vaccinia virus is conserved among all sequenced members of the Poxviridae but has no predicted function or recognized nonpoxvirus homolog. Here we provide the initial characterization of the L5 protein. L5 is expressed following DNA replication with kinetics typical of a viral late protein, contains a single intramolecular disulfide bond formed by the virus-encoded cytoplasmic redox pathway, and is incorporated into intracellular mature virus particles, where it is exposed on the membrane surface. To determine whether L5 is essential for virus replication, we constructed a mutant that synthesizes L5 only in the presence of an inducer. The mutant exhibited a conditional-lethal phenotype, as cell-to-cell virus spread and formation of infectious progeny were dependent on the inducer. Nevertheless, all stages of replication occurred in the absence of inducer and intracellular and extracellular progeny virions appeared morphologically normal. Noninfectious virions lacking L5 could bind to cells, but the cores did not enter the cytoplasm. In addition, virions lacking L5 were unable to mediate low-pH-triggered cell-cell fusion from within or without. The phenotype of the L5R conditional lethal mutant is identical to that of recently described mutants in which expression of the A21, A28, and H2 genes is repressed. Thus, L5 is the fourth component of the poxvirus cell entry/fusion apparatus that is required for entry of both the intracellular and extracellular infectious forms of vaccinia virus.
Figures



Similar articles
-
Vaccinia virus A21 virion membrane protein is required for cell entry and fusion.J Virol. 2005 Aug;79(15):9458-69. doi: 10.1128/JVI.79.15.9458-9469.2005. J Virol. 2005. PMID: 16014909 Free PMC article.
-
Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion.J Virol. 2005 Apr;79(8):4744-54. doi: 10.1128/JVI.79.8.4744-4754.2005. J Virol. 2005. PMID: 15795260 Free PMC article.
-
Entry of vaccinia virus and cell-cell fusion require a highly conserved cysteine-rich membrane protein encoded by the A16L gene.J Virol. 2006 Jan;80(1):51-61. doi: 10.1128/JVI.80.1.51-61.2006. J Virol. 2006. PMID: 16352530 Free PMC article.
-
Poxvirus entry and membrane fusion.Virology. 2006 Jan 5;344(1):48-54. doi: 10.1016/j.virol.2005.09.037. Virology. 2006. PMID: 16364735 Review.
-
Poxvirus host cell entry.Curr Opin Virol. 2012 Feb;2(1):20-7. doi: 10.1016/j.coviro.2011.11.007. Epub 2011 Dec 27. Curr Opin Virol. 2012. PMID: 22440962 Review.
Cited by
-
Three Conserved Regions in Baculovirus Sulfhydryl Oxidase P33 Are Critical for Enzymatic Activity and Function.J Virol. 2017 Nov 14;91(23):e01158-17. doi: 10.1128/JVI.01158-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28904203 Free PMC article.
-
Monkeypox: epidemiology, pathogenesis, treatment and prevention.Signal Transduct Target Ther. 2022 Nov 2;7(1):373. doi: 10.1038/s41392-022-01215-4. Signal Transduct Target Ther. 2022. PMID: 36319633 Free PMC article. Review.
-
Role of the vaccinia virus O3 protein in cell entry can be fulfilled by its Sequence flexible transmembrane domain.Virology. 2013 Sep;444(1-2):148-57. doi: 10.1016/j.virol.2013.06.003. Epub 2013 Jun 29. Virology. 2013. PMID: 23816434 Free PMC article.
-
Membrane fusion during poxvirus entry.Semin Cell Dev Biol. 2016 Dec;60:89-96. doi: 10.1016/j.semcdb.2016.07.015. Epub 2016 Jul 14. Semin Cell Dev Biol. 2016. PMID: 27423915 Free PMC article. Review.
-
Resistance of a vaccinia virus A34R deletion mutant to spontaneous rupture of the outer membrane of progeny virions on the surface of infected cells.Virology. 2007 Sep 30;366(2):424-32. doi: 10.1016/j.virol.2007.05.015. Epub 2007 Jun 5. Virology. 2007. PMID: 17553539 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

