Nogo-B is a new physiological substrate for MAPKAP-K2
- PMID: 16095439
- PMCID: PMC1276943
- DOI: 10.1042/BJ20050935
Nogo-B is a new physiological substrate for MAPKAP-K2
Abstract
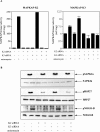
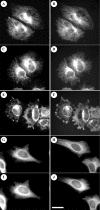
The neurite outgrowth inhibitor protein Nogo is one of 300 proteins that contain a reticulon homology domain, which is responsible for their association with the endoplasmic reticulum. Here we have found that the Nogo-B spliceform becomes phosphorylated at Ser107 in response to lipopolysaccharide in RAW264 macrophages or anisomycin in HeLa cells. The phosphorylation is prevented by SB 203580, an inhibitor of SAPK2a (stress-activated protein kinase 2a)/p38a and SAPK2b/p38b, and does not occur in embryonic fibroblasts generated from SAPK2a/p38a-deficient mice. Nogo-B is phosphorylated at Ser107 in vitro by MAPKAP-K2 [MAPK (mitogen-activated protein kinase)-activated protein kinase-2] or MAPKAP-K3, but not by other protein kinases that are known to be activated by SAPK2a/p38a. The anisomycin-induced phosphorylation of Ser107 in HeLa cells can be prevented by 'knockdown' of MAPKAP-K2 using siRNA (small interfering RNA). Taken together, our results identify Nogo-B as a new physiological substrate of MAPKAP-K2.
Figures



Similar articles
-
Inhibition of SAPK2a/p38 prevents hnRNP A0 phosphorylation by MAPKAP-K2 and its interaction with cytokine mRNAs.EMBO J. 2002 Dec 2;21(23):6505-14. doi: 10.1093/emboj/cdf639. EMBO J. 2002. PMID: 12456657 Free PMC article.
-
The phosphorylation of CapZ-interacting protein (CapZIP) by stress-activated protein kinases triggers its dissociation from CapZ.Biochem J. 2005 Jul 1;389(Pt 1):127-35. doi: 10.1042/BJ20050387. Biochem J. 2005. PMID: 15850461 Free PMC article.
-
Identification of glycogen synthase as a new substrate for stress-activated protein kinase 2b/p38beta.Biochem J. 2004 Apr 1;379(Pt 1):133-9. doi: 10.1042/BJ20031559. Biochem J. 2004. PMID: 14680475 Free PMC article.
-
Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB.EMBO J. 1998 Aug 3;17(15):4426-41. doi: 10.1093/emboj/17.15.4426. EMBO J. 1998. PMID: 9687510 Free PMC article.
-
Identification of anisomycin-activated kinases p45 and p55 in murine cells as MAPKAP kinase-2.Oncogene. 1996 Feb 15;12(4):805-12. Oncogene. 1996. PMID: 8632902
Cited by
-
Inhibition of Mitogen Activated Protein Kinase Activated Protein Kinase II with MMI-0100 reduces intimal hyperplasia ex vivo and in vivo.Vascul Pharmacol. 2012 Jan-Feb;56(1-2):47-55. doi: 10.1016/j.vph.2011.07.008. Epub 2011 Oct 17. Vascul Pharmacol. 2012. PMID: 22024359 Free PMC article.
-
p38 Mitogen-activated protein kinase regulates myelination.J Mol Neurosci. 2008 May;35(1):23-33. doi: 10.1007/s12031-007-9011-0. Epub 2007 Nov 10. J Mol Neurosci. 2008. PMID: 17994198 Review.
-
Platelet procoagulant phenotype is modulated by a p38-MK2 axis that regulates RTN4/Nogo proximal to the endoplasmic reticulum: utility of pathway analysis.Am J Physiol Cell Physiol. 2018 May 1;314(5):C603-C615. doi: 10.1152/ajpcell.00177.2017. Epub 2018 Feb 7. Am J Physiol Cell Physiol. 2018. PMID: 29412690 Free PMC article.
-
Evidence supporting changes in Nogo-B levels as a marker of neointimal expansion but not adaptive arterial remodeling.Vascul Pharmacol. 2007 Apr;46(4):293-301. doi: 10.1016/j.vph.2006.11.003. Epub 2006 Nov 18. Vascul Pharmacol. 2007. PMID: 17207665 Free PMC article.
-
Identification and regulation of reticulon 4B (Nogo-B) in renal tubular epithelial cells.Am J Pathol. 2010 Dec;177(6):2765-73. doi: 10.2353/ajpath.2010.100199. Epub 2010 Oct 22. Am J Pathol. 2010. PMID: 20971739 Free PMC article.
References
-
- Chen M. S., Huber A. B., van der Haar M. E., Frank M., Schnell L., Spillmann A. A., Christ F., Schwab M. E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature (London) 2000;403:434–439. - PubMed
-
- Fournier A. E., Strittmatter S. M. Repulsive factors and axon regeneration in the CNS. Curr. Opin. Neurobiol. 2001;11:89–94. - PubMed
-
- McKerracher L., Winton M. J. Nogo on the go. Neuron. 2002;36:345–348. - PubMed
-
- Spillmann A. A., Bandtlow C. E., Lottspeich F., Keller F., Schwab M. E. Identification and characterization of a bovine neurite growth inhibitor (bNI-220) J. Biol. Chem. 1998;273:19283–19293. - PubMed
-
- Kim J. E., Li S., GrandPre T., Qiu D., Strittmatter S. M. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

